Composite solid electrolyte prepared based on in-situ thermal polymerization method and preparation method and application thereof
A solid-state electrolyte and thermal polymerization technology, which can be used in composite electrolytes, electrolyte battery manufacturing, electrolytes, etc., can solve problems such as increasing the production cost of lithium-ion batteries, achieve good application prospects, facilitate migration, and improve the effect of utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
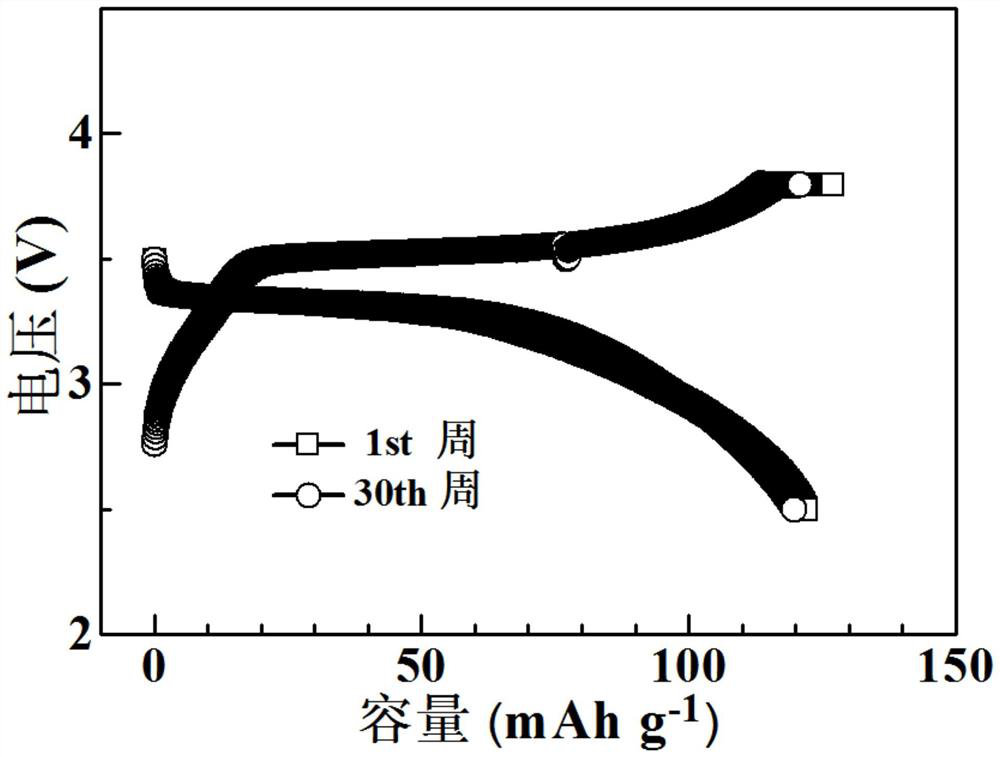
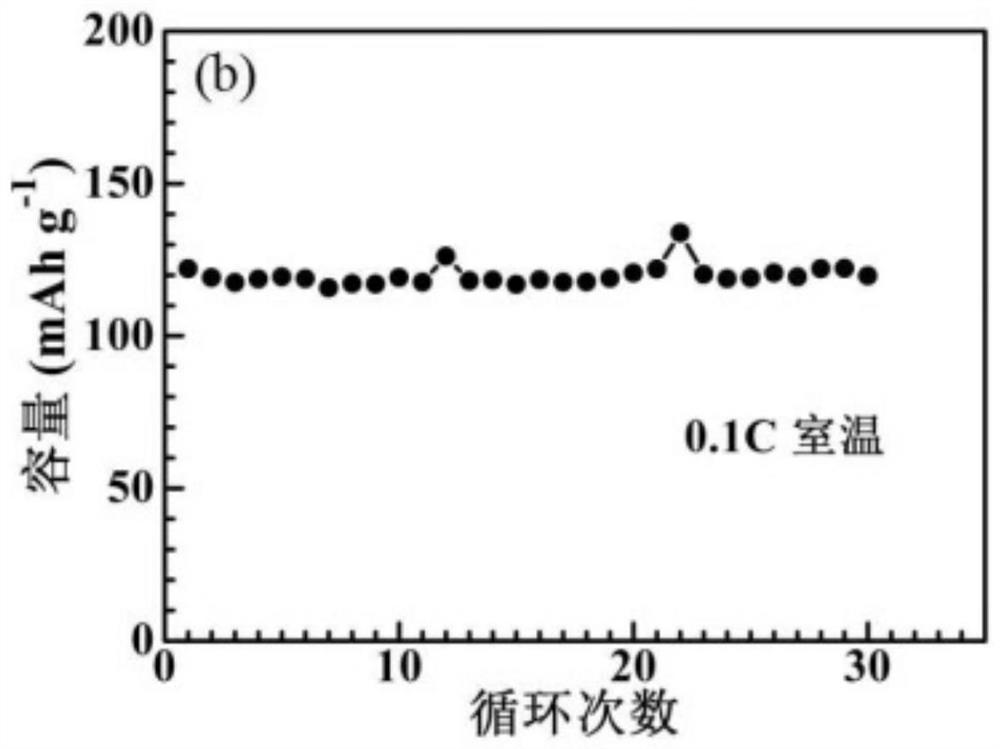
[0038]In an argon glove box, mix 2 g of lithium difluorooxalate borate LiDFOB, 0.01 g of azobisisobutyronitrile AIBN, 10 ml of vinylene carbonate and 3 ml of ethylene carbonate EC, stir magnetically until completely dissolved, and then add 2 g garnet-type oxide electrolyte Li 6.4 La 3 Zr 1.4 Ta 0.6 o 12 , Magnetic stirring to disperse evenly in the solution.
[0039] Lithium-ion button batteries were assembled in an argon glove box, and the positive electrode was made of lithium iron phosphate, Li 6.4 La 3 Zr 1.4 Ta 0.6 o 12 , Super P and PVDF are composed in a mass ratio of 75:9:10:6, and the negative electrode is lithium metal. During the assembly process, 1 ml of the above electrolyte solution is added, and the seal is sealed after the assembly is completed. The battery was left standing at room temperature for 12 hours to ensure that the electrolyte and electrode materials were fully infiltrated, and then kept at 60°C for 6 hours to allow in-situ polymerization of...
Embodiment 2
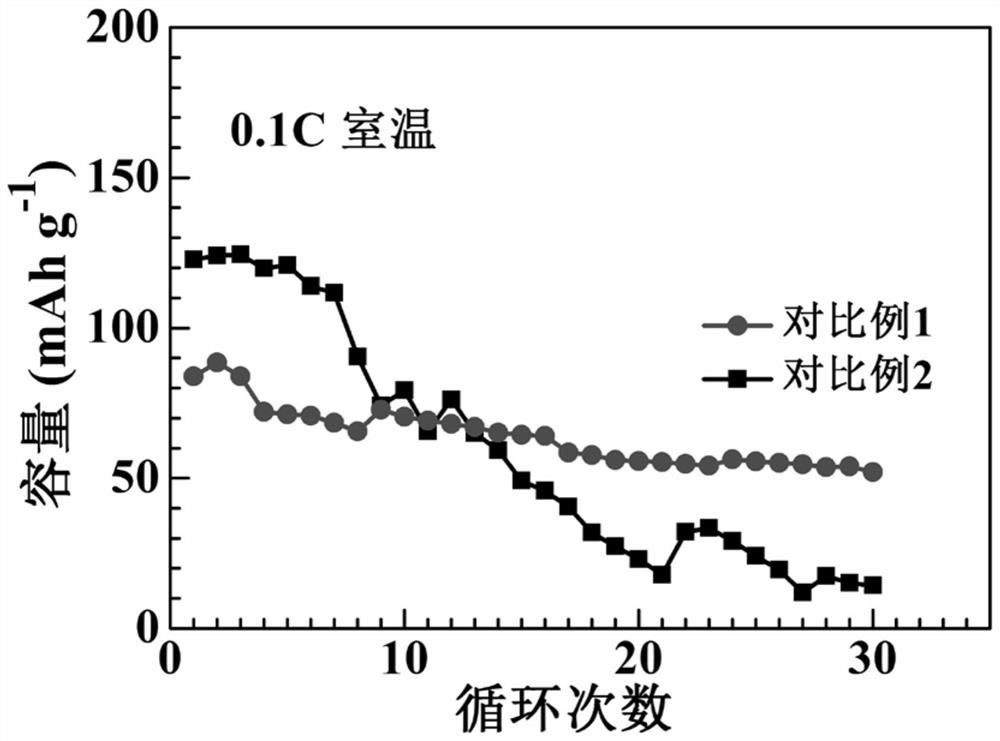
[0041] In an argon glove box, mix 1 g of lithium difluorooxalate borate LiDFOB, 1 g of lithium bistrifluoromethanesulfonimide LiTFSI, 0.02 g of benzoyl peroxide BPO, 10 mL of vinylene carbonate, and 1.6 mL of carbonic acid Methyl ethyl ester EMC mixed, magnetically stirred until completely dissolved, then added 3.5 grams of sulfide electrolyte 70Li 2 S·30P 2 S 5 , Magnetic stirring to disperse evenly in the solution.
[0042] Assemble a lithium-ion button battery in an argon glove box, and the positive electrode is made of lithium iron phosphate, 70Li 2 S·30P 2 S 5 , Super P and PVDF are composed in a mass ratio of 75:9:10:6, and the negative electrode is lithium metal. During the assembly process, 1 ml of the above electrolyte solution is added, and the seal is sealed after the assembly is completed. The battery was left standing at room temperature for 24 hours to ensure that the electrolyte and electrode materials were fully infiltrated, and then kept at 50°C for 24 ho...
Embodiment 3
[0044] In an argon glove box, 2 grams of lithium difluorooxalate borate LiDFOB, 2 grams of lithium hexafluorophosphate LiPF 6 , 0.02 g of azobisisoheptanonitrile ABVN, 10 ml of vinylene carbonate and 4 ml of dimethyl carbonate DMC were mixed, magnetically stirred until completely dissolved, and then 6 g of NASICON type electrolyte Li was added 1.3 al 0.3 Ti 1.7 P 3 o 12 , Magnetic stirring to disperse evenly in the solution.
[0045] Lithium-ion button batteries were assembled in an argon glove box, and the positive electrode was made of lithium iron phosphate, Li 1.3 al 0.3 Ti 1.7 P 3 o 12 , Super P and PVDF are composed in a mass ratio of 75:9:10:6, and the negative electrode is lithium metal. During the assembly process, 1 ml of the above electrolyte solution is added, and the seal is sealed after the assembly is completed. The battery was left to stand at room temperature for 18 hours to ensure that the electrolyte and electrode materials were fully infiltrated, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Sub-conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com