Cu2O/N-C oxygen reduction catalyst and preparation and application thereof
A catalyst, cuprous oxide technology, applied in the field of material chemistry, can solve the problems of poor stability of metal nitrogen carbon materials, damage to battery materials and devices, shortage of metal platinum resources, etc., achieve excellent stability, solve poor stability, and improve electrochemical performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
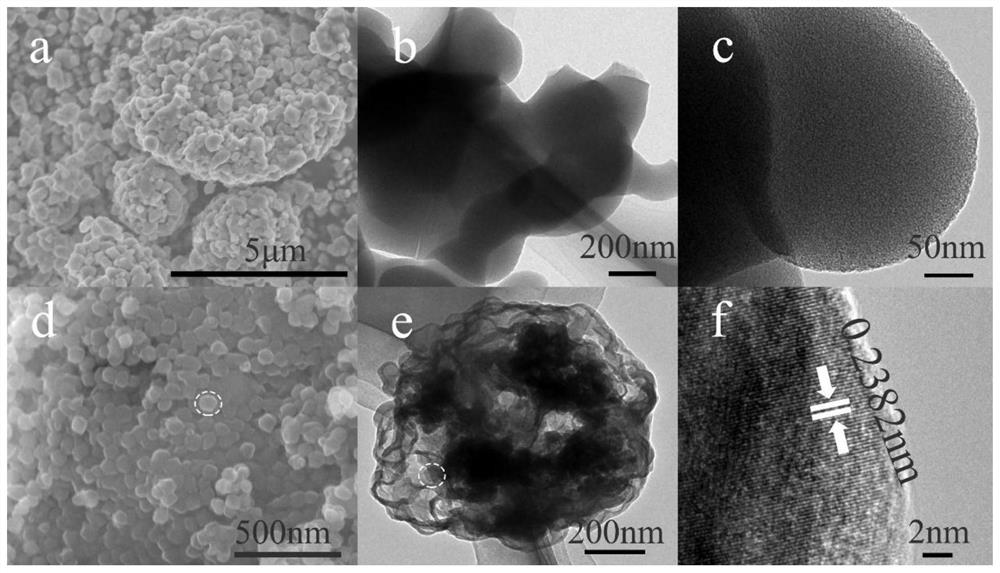
[0064] First weigh 2.30g of 2-methylimidazole (28mmoL) and 20mg of CuPC (copper phthalocyanine, 0.035mmoL) and dissolve them in 40ml of methanol, stir until completely dissolved, and weigh 1.00g of Zn(NO 3 ) 2 ·6H 2 O (3.6mmoL) was dissolved in another 40ml of methanol, stirred until completely dissolved, Zn(NO 3 ) 2 ·6H 2 O methanol solution was slowly added to 2-methylimidazole and 20mg CuPC methanol solution, stirred at 30°C at 500-550rmp for 24h, centrifuged at 5000rmp for 5min, washed twice with methanol, and placed in an oven at 80°C Dry in medium. Put the dried samples into a porcelain boat, 2 The above samples were sintered at high temperature in the atmosphere, the heating rate was 10°C / min, the temperature was controlled at 800°C, and the holding time was 3h. The sample obtained by the above steps is named Cu 2 O / N-C-1.
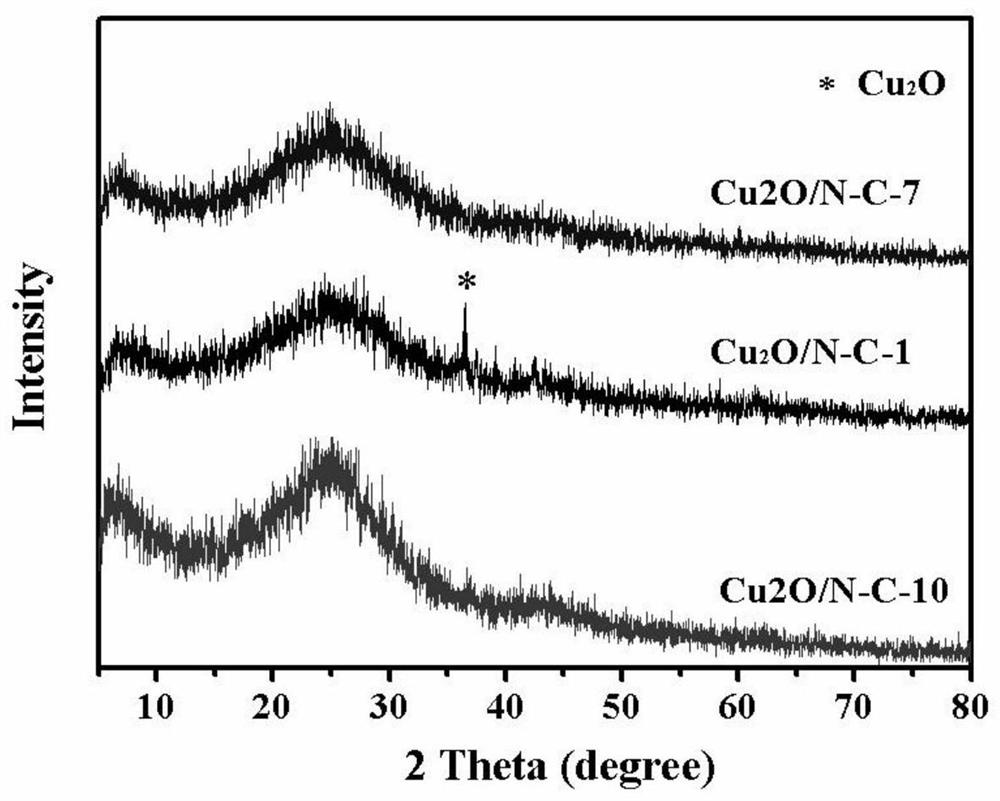
[0065] Adopt X-ray diffractometer (XRD, Rigaku-D / Max 2550, Cu-Kα, 40kV, 300mA) to carry out phase analysis to the product. The morphology ...
Embodiment 2
[0068] Compared with Example 1, the difference is that the consumption of CuPC is reduced, specifically as follows:
[0069] First weigh 2.30g of 2-methylimidazole and 16mg of CuPC (0.028mmoL) and dissolve them in 40ml of methanol, stir until completely dissolved, and weigh 1.00g of Zn(NO 3 ) 2 ·6H 2 O was dissolved in another 40ml of methanol, stirred until completely dissolved, Zn(NO 3 ) 2 ·6H 2 O methanol solution was slowly added to 2-methylimidazole and 16mg CuPC methanol solution, stirred at 30°C at 500-550rmp for 24h, centrifuged at 5000rmp for 5min, washed twice with methanol, and placed in an oven at 80°C Dry in medium. Put the dried samples into a porcelain boat, 2 The above samples were sintered at high temperature in the atmosphere, the heating rate was 10°C / min, the temperature was controlled at 800°C, and the holding time was 3h. The sample obtained by the above steps is named Cu 2 O / N-C-2.
[0070] The ORR performance test of the material and the prepar...
Embodiment 3
[0072] Compared with Example 1, the difference is that the consumption of CuPC is increased, specifically as follows:
[0073] First weigh 2.30g of 2-methylimidazole and 26mg of CuPC (0.046mmoL) and dissolve them in 40ml of methanol, stir until completely dissolved, and weigh 1.00g of Zn(NO 3 ) 2 ·6H 2 O was dissolved in another 40ml of methanol, stirred until completely dissolved, Zn(NO 3 ) 2·6H 2 O methanol solution was slowly added to 2-methylimidazole and 26mg CuPC methanol solution, stirred at 500-550rmp at 30°C for 24h, centrifuged at 5000rmp for 5min, washed twice with methanol, and placed in an oven at 80°C Dry in medium. Put the dried samples into a porcelain boat, 2 The above samples were sintered at high temperature in the atmosphere, the heating rate was 10°C / min, the temperature was controlled at 800°C, and the holding time was 3h. The sample obtained by the above steps is named Cu 2 O / N-C-3.
[0074] The ORR performance test of the material and the prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com