Nano antibody for neutralizing toxicity of novel coronavirus as well as preparation method and application of nano antibody
A nanobody and virus technology, applied in the field of peptides, can solve the problems of limited sources and mismatch of blood types of donors and recipients, and achieve the effects of low production cost, strong neutralization ability, and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
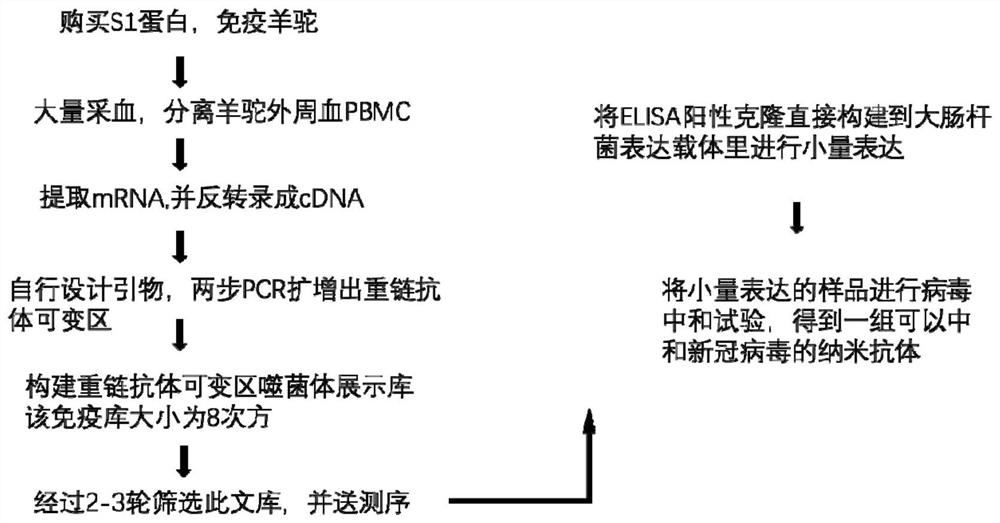
[0056] Example 1 Preparation of New Coronavirus Neutralizing Toxicity Nanobodies
[0057] 1. Animal immunization program and results
[0058] Alpaca (llama) immunization program: an adult male healthy llama alpaca, number KZL007, is immunized by subcutaneous injection at multiple points. 0.9 mg of S1 protein was injected each time, once every 14 days, for a total of 3 injections. 14 days after the third immunization, 2 ml of blood was collected to measure the titer.
[0059] llama immune titer detection method: using enzyme-linked immunosorbent assay, the target antigen is the new coronavirus RBD protein, the primary antibody is anti-his-HRP, TMB color development, 450nm detection O.D. value). When the serum is diluted 128K times (ie, 128000 times, the meaning of the expression is similar in the following text), the ELISA result can be above 1.0. During detection, the serum was diluted with pH 7.4 PBS buffer, wherein the serum was diluted by 1K times, 2K times, 4K times, 8K...
Embodiment 2
[0122] Example 2 Affinity detection test of novel coronavirus neutralizing toxic nanobodies
[0123] 1. Instruments and reagents:
[0124] In this example, the instrument Gator probe life is used to detect the intermolecular interaction by thin film interference method, and the sample to be tested with a concentration of not less than 80% and the antigen RBD-FC protein (new coronavirus RBD protein) of a concentration of not less than 90% are prepared .
[0125] Probe species: Kinetic index of binding of anti-human Fc antibody and human Fc.
[0126] Buffer: Q buffer (PBS+0.02%Tween-20+0.2%BSA).
[0127] 2. Experimental method:
[0128] 1) NBS1-2 sample detection method: immobilized antigen RBD-Fc (100nM) to detect nanobody; probe type: anti-human Fc antibody; NBS1-2 was diluted to 60000nM, 30000nM, 15000nM, 7500nM, 3750nM with Q buffer , 1875nM, 937.5nM, OnM.
[0129] 2) NBS1-3 sample detection method: immobilized antigen RBD-Fc (100nM) to detect nanobody; probe type: anti...
Embodiment 3
[0137] Example 3 Virus Neutralization Test of Novel Coronavirus Neutralizing Toxicity Nanobodies
[0138] The virus neutralization test is a technology for detecting specific antibodies, that is, the interference ability of the biological activity of the antibody to be tested is determined by using a known virus. In the embodiment of the present invention, this technology is used to detect the activity of the screened nanobody.
[0139] Four nanobody samples with a purity of more than 80% were prepared: NBS1-2, NBS1-3, NBS1-10 and NBS1-57; the nanobody used was prepared in Example 1.
[0140] 1. Experimental method:
[0141] 1) First, take the original concentration of each protein sample as the initial concentration, use the sample diluent (20mM PB, pH7.2) to dilute each protein sample in a gradient, set 1mg / ml, 100μg / ml, 10μg / ml, 1μg in turn / ml, 0.1μg / ml, 0.01μg / ml and other dilution concentrations;
[0142] 2) The diluted sample was incubated with an equal volume of SARS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com