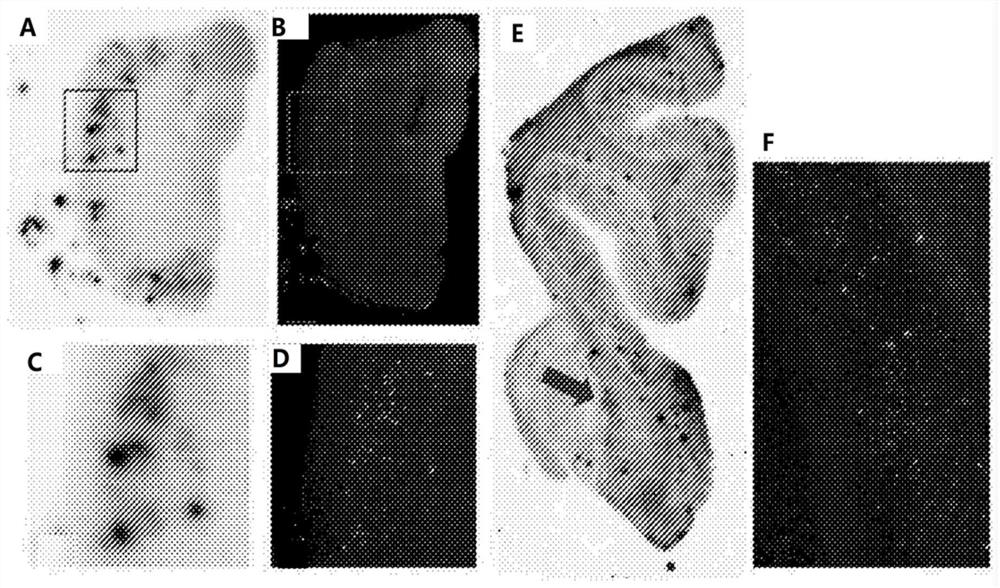
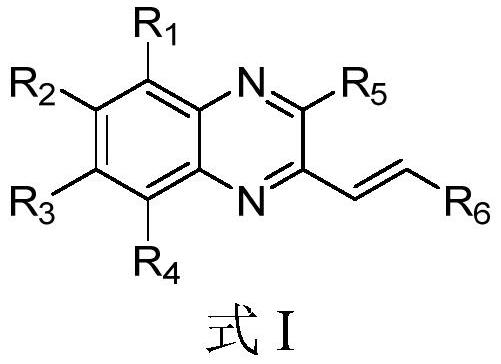
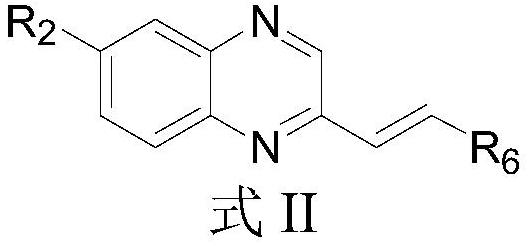
Pyridine derivative and preparation method and application thereof
A compound and hydrate technology, applied in the field of pyridine derivatives and its preparation, can solve problems such as unsatisfactory effects and difficult synthesis of Tau protein tracers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1, the synthesis of compound 5 of the present invention
[0061] (1) Synthetic compound 3:
[0062]
[0063] Compound 1 (22.0g, 143.66mmol) and compound 2 (10.35g, 143.66mmol) were dissolved in glacial acetic acid, refluxed for 5 hours, after the reaction was monitored by TLC, the solvent was spin-dried, washed with water, extracted with EA (ethyl acetate) , the organic phase was dried with anhydrous sodium sulfate, filtered, concentrated, and then subjected to silica gel column chromatography to obtain compound 3 (23 g, yield 85%). MS(ESI)m / z:190.1[M+H] + .
[0064] (2) Synthetic compound 5:
[0065]
[0066] Methyl trioctyl ammonium chloride (Aliquat336, 64mg, 0.159mmol) was dissolved in 5M sodium hydroxide solution, compound 3 (150.00mg, 0.797mmol), 5-dimethylaminothiophene-2-formaldehyde (compound 4, 123mg, 0.797mmol), reflux at 110°C, after TLC monitors the reaction is complete, adjust the pH to 7, wash with water, extract with DCM, take the or...
Embodiment 2
[0067] Embodiment 2, the synthesis of compound 6 of the present invention
[0068]
[0069] A solution of compound 5 (30 mg) and the phase transfer catalyst kryptofix (5 mg) in DMF (1.5 mL) was added to a solution containing dry [ 18 F] fluoride (this embodiment is K[ 18 F]) in a microwave reaction tube, and the reaction mixture was heated at 140°C (65W) for 4 minutes. After cooling below 50°C, the reaction mixture was washed with H 2 0 (1 mL), mixed, and injected onto a semi-preparative HPLC column. The resulting product was purified using a Zorbax Eclipse XDB-C-18 liquid chromatography column, 5 μm, 9.4×250 mm (Agilent) at a flow rate of 5 mL / min. The mobile phase used was 50% to 80% acetonitrile solution, and the solvent in this solution was 10 mM NaH 2 P0 4 aqueous solution for 15 minutes. The resulting radioactive fraction was collected, concentrated under reduced pressure, diluted with 0.9% saline solution (3 mL) and transferred to a sterile container to obtain ...
Embodiment 3
[0071] Embodiment 3, the synthesis of compound 7~11 of the present invention
[0072] According to the synthetic route of the above-mentioned general formula compound 5a, adopt the same method as in Example 1, the compound 4 in Replace with the corresponding R 6 group to prepare compounds 7-11 of the present invention, the structures and characterizations of which are shown in Table 1.
[0073] Table 1 Structure and characterization data of compounds 7-11 of the present invention
[0074]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com