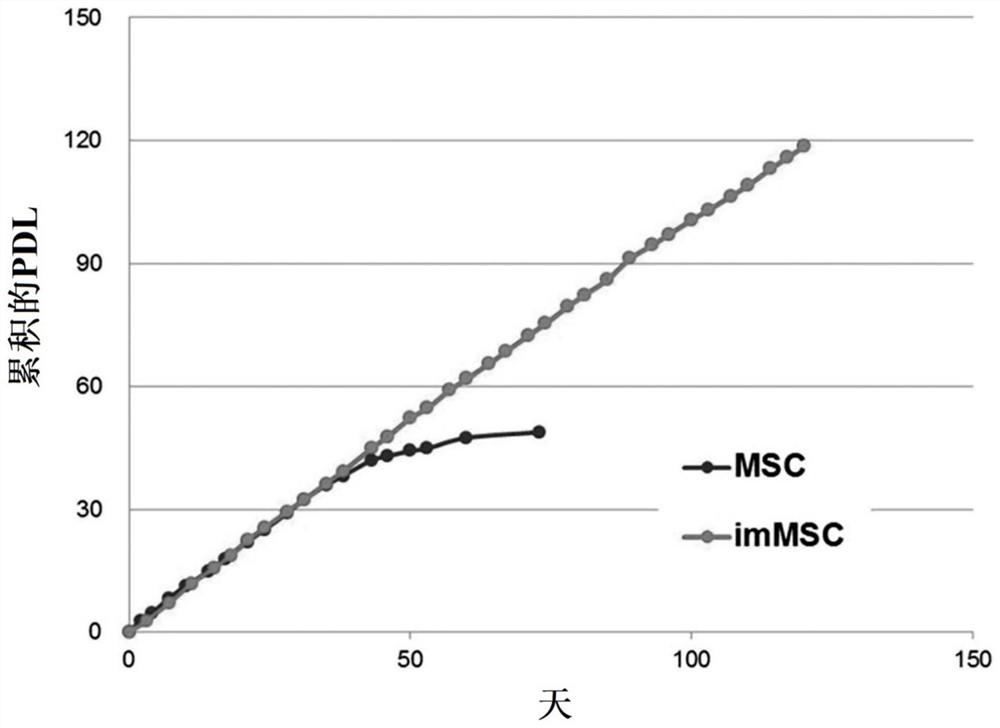
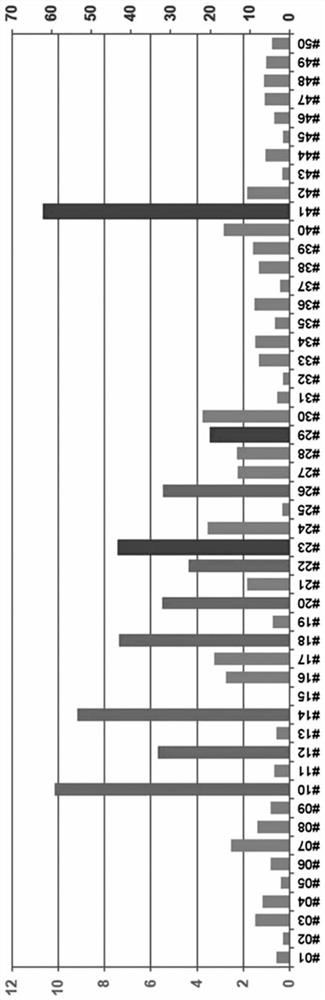
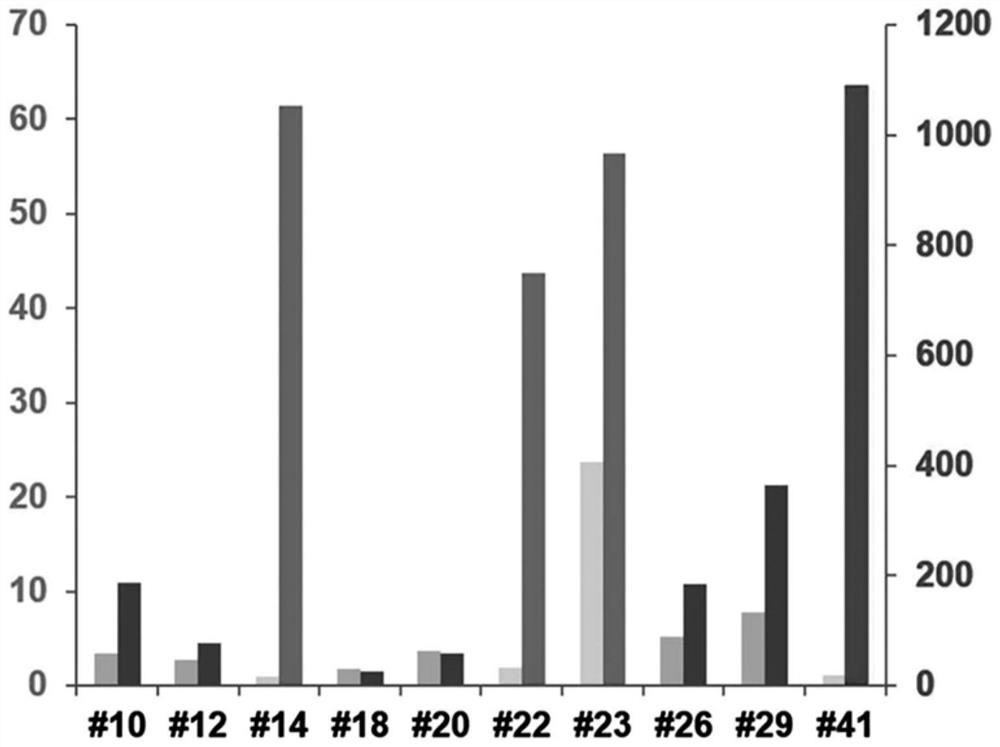
Mesenchymal stem cells expressing brain-derived neurotrophic factor and use thereof
A neurotrophic factor and brain-derived technology, applied to genetically modified cells, cells modified by introducing foreign genetic material, animal cells, etc., can solve the problems of difficult mass production of MSCs, difficult to expect effects, limited proliferation, etc., and achieve Possibility of differentiation and proliferation is low, the formation of brain tissue is promoted, and the effect of high cell proliferation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1. Preparation of immortalized mesenchymal stem cells (MSCs)
Embodiment 11
[0102] Example 1.1. Preparation of a lentiviral vector comprising an immortalized gene
[0103] To immortalize MSCs, lentiviral vectors containing c-Myc and / or hTERT, respectively, as immortalization genes were prepared. At this time, in order to use the Tet-off system, a gene construct expressing tTA protein was inserted together.
[0104] First, in the expression cassette of the pWPT vector (Addgene, USA), the EF promoter was replaced with the CMV promoter, and the RSV promoter was additionally connected downstream to prepare the pBD lentiviral vector.
[0105] Using IRES, the c-Myc gene (SEQ ID NO: 6) and the thymidine kinase (TK) gene (SEQ ID NO: 4) were connected and inserted into the pBD lentiviral vector to regulate expression according to the CMV promoter. The vector prepared above was named pBD-1.
[0106] On the other hand, the hTERT gene (SEQ ID NO: 8) was inserted into the pBD lentiviral vector for regulated expression according to the CMV promoter. Among them, ...
Embodiment 12
[0108] Example 1.2. Production of Lentiviruses Comprising Immortalization Genes
[0109] Using the lentiviral vector prepared in the above Example 1.1., the lentivirus containing the immortalization gene was produced by the following method.
[0110] First, Lenti-X cells (Clontech Laboratories, USA) were cultured in 150 mm dishes (dish) using DMEM medium containing 10% fetal bovine serum. On the other hand, lentiviral vectors were extracted and quantified from DH5α E. coli cells using EndoFree PlasminMaxi Kit (Qiagen, USA).
[0111] After washing the above cultured Lenti-X cells with PBS, add 3ml of TrypLE TM Select CTS TM(Gibco, USA). After leaving the cells at 37° C. for about 5 minutes, it was confirmed that the cells were desorbed. The desorbed cells were added to DMEM medium containing 7 ml of 10% fetal calf serum for neutralization. The neutralized cells were aggregated in a 50ml test tube and centrifuged at 1500rpm for 5 minutes. The supernatant was removed, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com