Mesenchymal stem cell expressing hepatocyte growth factor, and use thereof
A hepatocyte growth factor and host cell technology, applied in the field of recombinant lentiviral vectors, can solve the problems of mesenchymal stem cells, difficulty in mass production of MSCs, and MSCs proliferation limitation, etc., achieving high safety, The effect of blocking possibility and preventing tumor formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
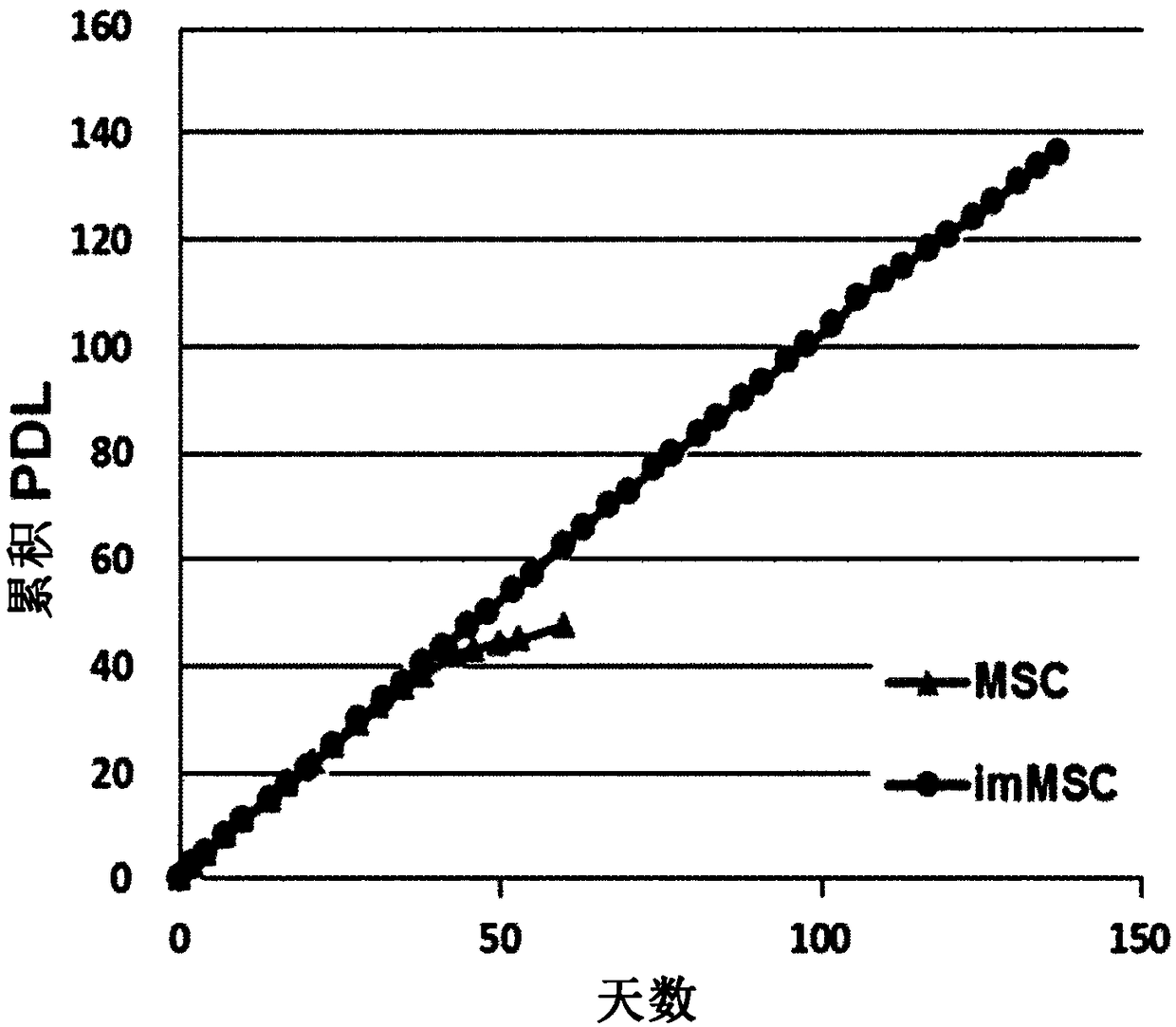
[0067] Example 1. Preparation of Immortalized Mesenchymal Stem Cells (MSCs)
[0068] 1-1. Preparation of lentiviral vector containing immortalization gene
[0069] To immortalize MSCs, lentiviral vectors containing the immortalization genes c-Myc and hTERT, respectively, were prepared. Here, a gene construct expressing tTA protein was inserted together to use the Tet-off system.
[0070] First, the pBD lentiviral vector was prepared by replacing the EF promoter in the expression cassette of the pWPT vector (Addgene, USA) with the CMV promoter and adding the RSV promoter downstream of it.
[0071] c-Myc gene (SEQ ID NO: 6) and thymidine kinase (TK) gene (SEQ ID NO: 4) are linked by IRES, and then inserted into pBD lentiviral vector, so that the expression can be regulated by CMV promoter. The constructed vector was named pBD-1.
[0072] On the other hand, the hTERT gene (SEQ ID NO: 8) was inserted into the pBD lentiviral vector so that the expression could be regulated by th...
Embodiment 2
[0085] Example 2. Construction of lentivirus containing HGF gene
[0086] 2-1. Construction of lentiviral vector containing HGF gene
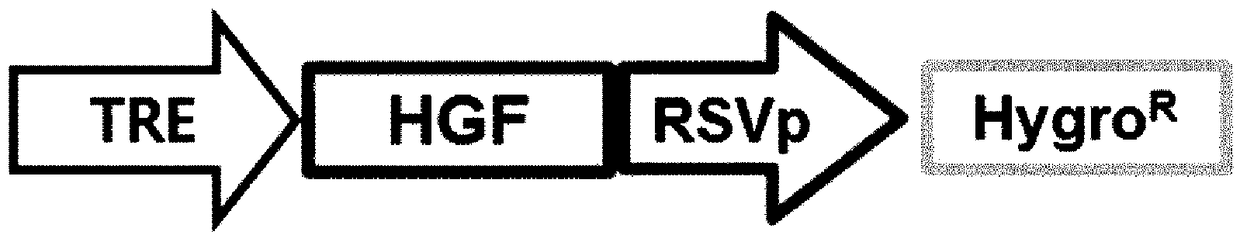
[0087] The HGF gene (SEQ ID NO: 2) was inserted into the pBD lentiviral vector generated above. Here, the inserted HGF gene was designed to be regulated by the TRE promoter. The TRE promoter can regulate the expression of genes linked to the TRE promoter depending on whether or not doxycycline is added.
[0088] Here, a gene for hygromycin resistance (HygroR; SEQ ID NO: 16) was inserted so that its expression could be regulated by the RSV promoter. The constructed vector was named pBD-4, and the structure of the gene construct was as follows: figure 2 shown.
[0089] 2-2. Generation of lentivirus containing HGF gene
[0090] Using the lentiviral vector containing the HGF gene constructed in Example 2-1, a lentivirus was prepared by the same method as described in Example 1-2. The resulting lentivirus was prepared as 3.5 × 10 8 Concentra...
Embodiment 3
[0091] Example 3. Preparation of MSC infected with lentivirus containing HGF gene
[0092] 3-1. Preparation of MSC transfected with lentivirus containing HGF gene
[0093] Cells expressing the HGF gene were prepared by infecting the immortalized MSCs prepared in Example 1-3 with the lentivirus constructed in Example 2-2 containing the HGF gene. Infection was performed by the same method as described in Examples 1-3. After infection, cells infected with pBD-4 lentivirus were selected by adding 25 μg / ml hygromycin to the medium of the stabilized cells. The selected cells were cultured in a medium supplemented with 2 μg / ml of doxycycline (631311, Clontech, USA) to suppress the expression of HGF protein during the culture.
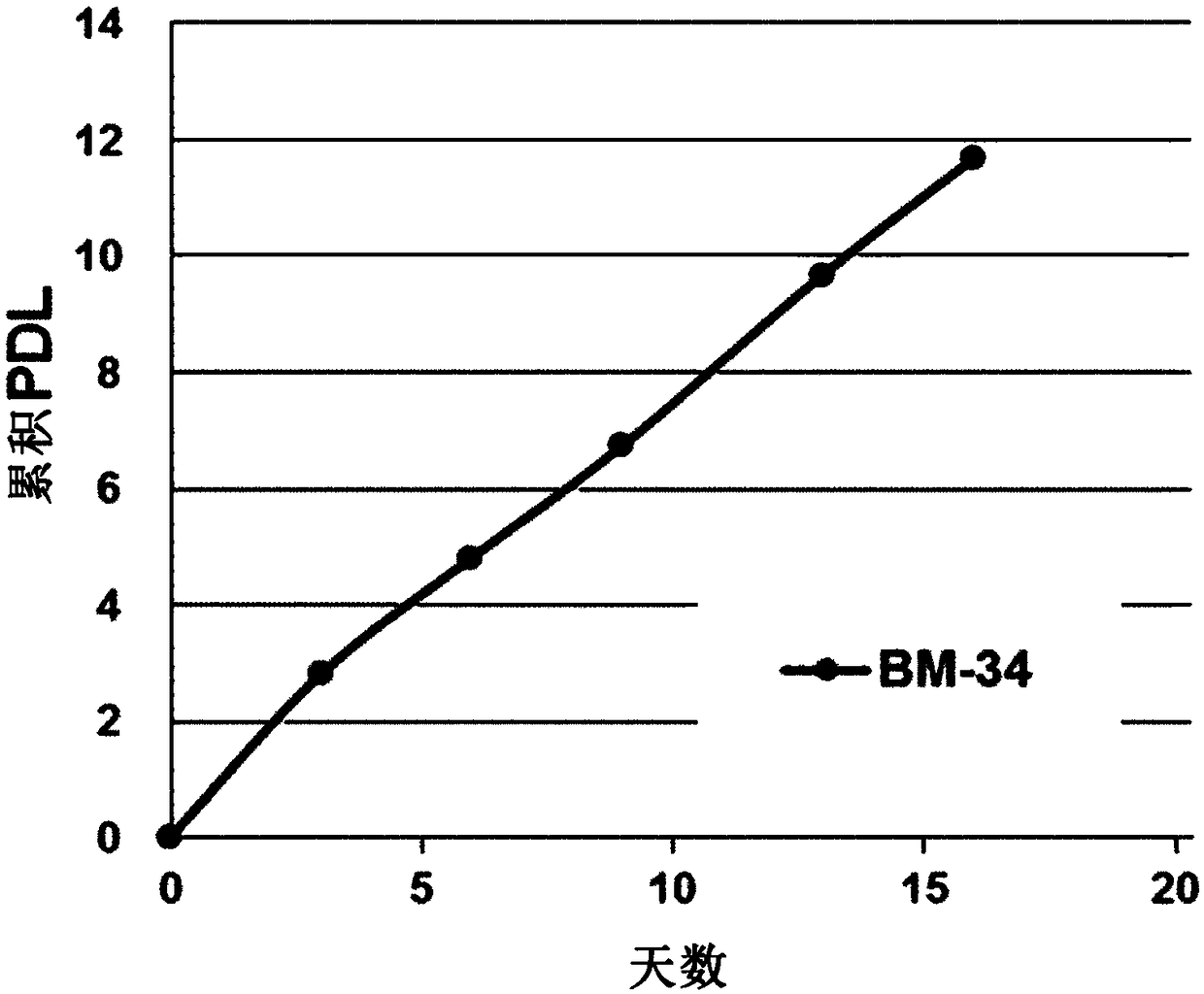
[0094] Selected cells are cultured to form clones. Monoclonal cells obtained from the formed clones were cultured to establish a cell line, which was named BM-34A. The cell line BM-34A was deposited on January 6, 2017 at the Korean Type Culture Collection ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com