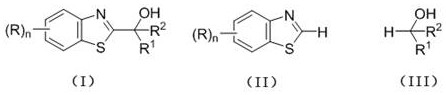
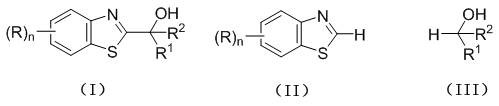
Preparation method of C2-substituted 2H-benzothiazole hydroxyalkylated derivative
A technology of benzothiazole hydroxyalkyl and benzothiazole, which is applied in the field of preparation of C2-substituted 2H-benzothiazole hydroxyalkylated derivatives, can solve problems such as high price of Selectfluor, and achieves easy availability of catalyst and simple catalytic system. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Derivative Ia ((R)n=H, R 1 = H, R 2 = methyl) synthesis
[0024] Weigh 2 H - Benzothiazole (0.3 mmol, 40.6 mg), ethanol (6.0 mmol, 276.4 mg) and 1.5 mol% Eosin B were placed in a 25 mL reaction tube, then 2 mL of acetonitrile was added, and placed under a 20 W power LED white light reaction at room temperature, TLC monitoring, after about 16 h, the reaction was completed, the reaction solution was concentrated to remove the solvent, and the concentrated solution was separated by column chromatography (the eluent was a mixed solvent of sherwood oil-ethyl acetate with a volume ratio of 1: 1 ) to obtain a yellow solid, the derivative Ia. Yield 78%.
[0025] of the compound 1 H NMR and 13 C NMR analysis data are described below,
[0026] 1 H NMR (500 MHz, CDCl 3 ) δ 7.96 (d, J = 8.0 Hz, 1H), 7.86 (d, J = 8.0 Hz,1H), 7.45 (dd, J = 8.0, 7.0 Hz, 1H), 7.36 (t, J = 8.0 Hz, 1H), 5.25 (q, J =6.5 Hz, 1H), 3.81 (br, 1H), 1.70 (d, J = 6.5 Hz, 3H). 13...
Embodiment 2
[0027] Example 2 Derivative Ib ((R)n=H, R 1 = H, R 2 = ethyl) synthesis
[0028] Weigh 2 H - Benzothiazole (0.3 mmol, 40.6 mg), n-propanol (6.0 mmol, 360.6 mg) and 3.0 mol% Eosin B were placed in a 25 mL reaction tube, then 2 mL of acetonitrile was added, and placed under a 20 W power LED white light Reaction under light irradiation, stirring reaction at room temperature, TLC monitoring, after about 18 h, the reaction was completed, the reaction solution was concentrated to remove the solvent, and the concentrated solution was separated by column chromatography (the eluent was petroleum ether-ethyl acetate with a volume ratio of 1:1 mixed solvent) to obtain a white solid, namely derivative Ib. The yield is 70%.
[0029] of the compound 1 H NMR and 13 C NMR analysis data are described below,
[0030] 1 H NMR (500 MHz, CDCl 3 ) δ 7.98 (d, J = 8.5 Hz, 1H), 7.88 (d, J = 8.0 Hz,1H), 7.50 – 7.44 (m, 1H), 7.41 – 7.35 (m, 1H), 5.05 (dd, J = 7.5, 5.0 Hz,1H), 3.70 (br, ...
Embodiment 3
[0031] Example 3 Derivative Ic ((R)n=H, R 1 = methyl, R 2 = methyl) synthesis
[0032] Weigh 2 H - Benzothiazole (0.3 mmol, 40.6 mg), isopropanol (3.0 mmol, 180.3 mg) and 1.5 mol% Eosin B in a 25 mL reaction tube, then add 2 mL of acetonitrile, and place under a 20 W LED white light Reaction, stirring reaction at room temperature, TLC monitoring, after about 16 h, the reaction ends, the reaction solution is concentrated to remove the solvent, and the concentrated solution is separated by column chromatography (the eluent is a mixed solvent of petroleum ether-ethyl acetate with a volume ratio of 1: 1) A white solid, the derivative Ic, was obtained. The yield is 85%.
[0033] of the compound 1 H NMR and 13 C NMR analysis data are described below,
[0034] 1 H NMR (500 MHz, CDCl 3 ) δ 8.00 (d, J = 8.0 Hz, 1H), 7.89 (d, J = 8.0 Hz,1H), 7.48 (t, J = 7.5 Hz, 1H), 7.38 (t, J = 7.0 Hz, 1H), 3.35 (br, 1H), 1.77(s, 6H). 13 C NMR (125 MHz, CDCl 3 ) δ 180.10, 153.05, 13...
PUM
| Property | Measurement | Unit |
|---|---|---|
| power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com