Time-resolved fluorescence immunochromatographic test strip for detecting influenza A virus, influenza B virus and novel coronavirus antigen and preparation method thereof
A technology of influenza B virus and immunochromatographic test paper, applied in the field of medical examination, can solve the problems of long cycle and complicated operation, and achieve the effect of simple operation and convenient clinical diagnosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Time-resolved immunochromatographic test strip for simultaneous detection of influenza A, B and novel coronavirus.
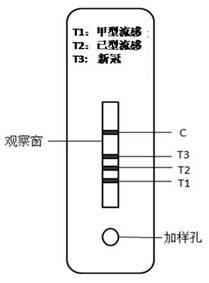
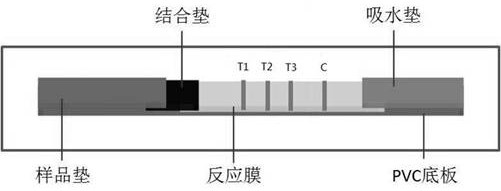
[0038] A time-resolved immunochromatographic test strip for simultaneous detection of influenza A and B viruses and novel coronavirus antigens in this embodiment includes a base plate and a sample pad, a binding pad, a reaction membrane and a water-absorbing pad sequentially arranged on the base plate pad. The binding pad is coated with an equivalent number of fluorescent microspheres labeled influenza A, B and novel coronavirus monoclonal detection antibodies; the reaction membrane includes parallel arrangements and 2mm and 4mm intervals between T1, T2, T3 detection area and control area; the T1 detection area is close to the binding pad, the control area is close to the water-absorbing pad, the T2 and T3 detection areas are located in the middle of the T1 detection area and the control area, and the T2 detection area is close to the T1 detecti...
Embodiment 2
[0048] Example 2 Time-resolved immunochromatographic kit for simultaneous detection of influenza A, B and novel coronavirus antigens.
[0049] The time-resolved fluorescent immunochromatography kit for simultaneous detection of influenza A and B viruses and novel coronavirus antigens in this embodiment, the kit includes: the test strip described in Embodiment 1, a plastic cartridge, and a sample buffer.
[0050] In this embodiment, the sample buffer is PBS buffer containing 0.5% BSA and 0.05% Tween-20, pH 7.4, 0.02mol / L.
[0051] When using the time-resolved fluorescence immunochromatography kit for simultaneous detection of influenza A, B and novel coronavirus antigens of the present invention, the throat swab sample to be tested is added to the sample buffer, and 60 µL of sample is added dropwise on the sample pad to dilute liquid, which transports the sample to the conjugate pad by capillary action. When the throat swab sample contains influenza A and (or) influenza B viru...
Embodiment 3
[0052] Performance measurement of the kit of Example 3.
[0053] The performance of the kit was determined, including the minimum detection limit, precision, sensitivity, specificity, etc.
[0054] 1. The lowest detection limit.
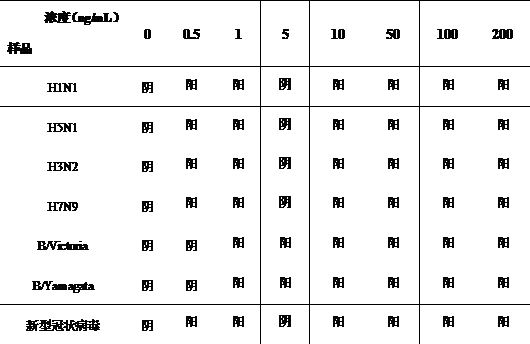
[0055] Its test results are shown in Table 1, and it can be seen from the table that the minimum detection limit of type A H1N1, type A H5N1, type A H3N2, and type A H7N9 is 0.5ng / mL; the minimum detection limit of B / Victoria and B / Yamagata is 1.0ng / mL; the minimum detection limit of the new coronavirus is 0.5ng / mL.
[0056] 2. Intra-batch precision: 10 time-resolved immunochromatography kits of the same batch number were randomly selected to detect the same concentration of influenza A, B and new coronavirus antigen reference products, and the coefficient of variation CV (%) Value ≤ 10%.
[0057] 3. Inter-batch precision: Randomly select three batches of time-resolved immunochromatography kits in a row, and take 3 copies from each batch to test t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com