Mutant protein for proliferating immune cells
A mutant and protein technology, applied in the field of protein engineering, can solve the problems of easy generation of multimers, unfavorable production and quality control, and mutual binding between different molecules.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
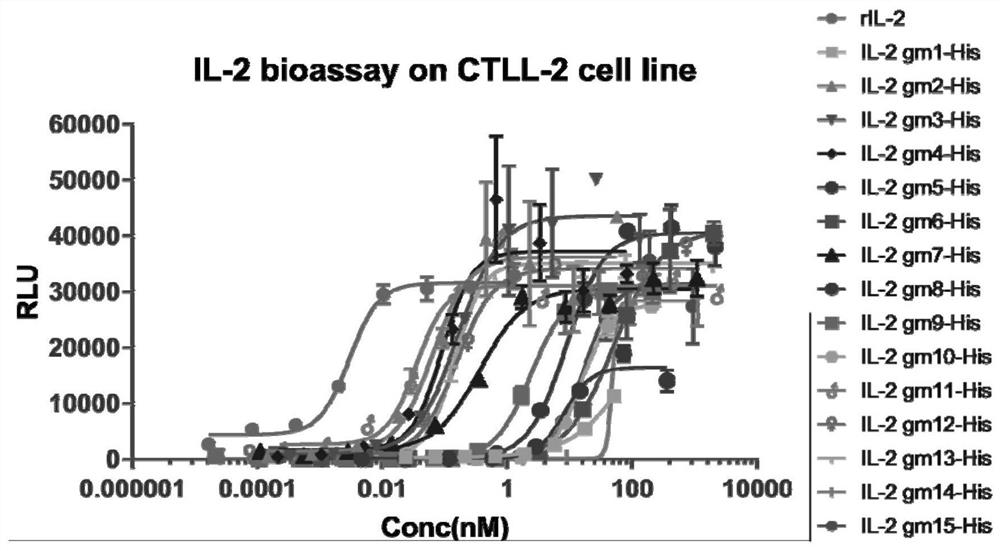
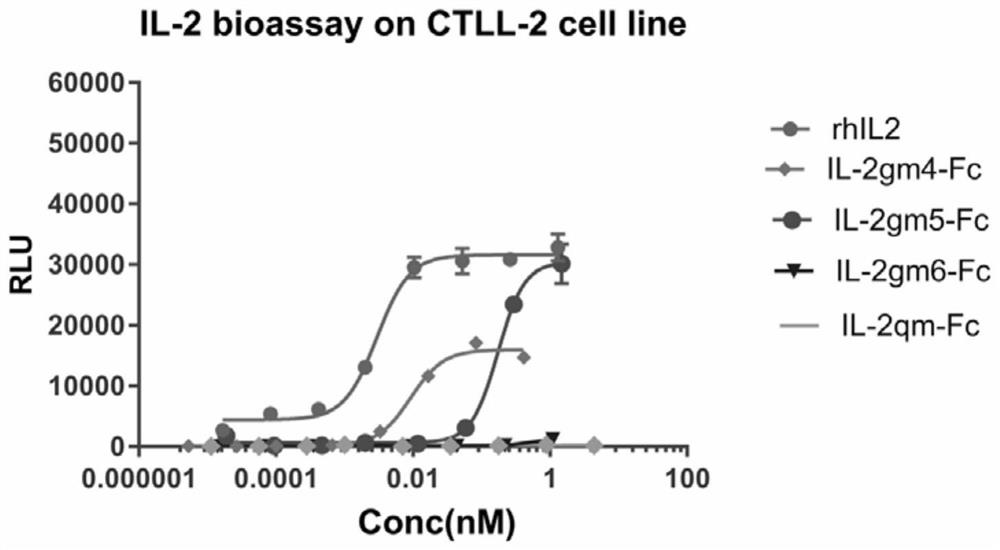
[0138] Example 1. Synthesis of Mutant Interleukin-2 (IL-2) Proteins
[0139] 1. Gene synthesis
[0140] The nucleotide sequence encoding the mutant amino acid sequence of the interleukin-2 (IL-2) protein is obtained by an automatic gene synthesis method. In some embodiments, adding HIS tags to the ends of gene fragments facilitates purification; in some embodiments, adding IgG1-Fc to the ends of gene fragments facilitates purification, and Fc tags are also a common means of extending the half-life of protein drugs. The gene fragment is flanked by a single restriction enzyme cleavage site. All gene synthesis sequences were designed with a 5' DNA sequence encoding a leader peptide that targets the protein for secretion in eukaryotic cells.
[0141]
[0142] 2. Plasmid construction
[0143] The synthesized gene was subcloned into the pcDNA3.4 plasmid using molecular biology reagents according to the manufacturer's instructions.
[0144] 3. Expression of mutant interleukin-...
Embodiment 2
[0147] Example 2.Expression of CD25 protein
[0148] gene synthesis
[0149] The nucleotide sequence encoding the amino acid sequence of CD25 protein (SEQ ID NO: 23) is obtained by automatic gene synthesis. SEQ ID NO: 24 (GGGSGGGSGGGSGGGS) is the amino acid sequence of the linker. In some embodiments, the gene fragment is co-expressed with IgG1-Fc through a linker, which is convenient for purification. The gene fragment is flanked by a single restriction enzyme cleavage site. All gene synthesis sequences were designed with a 5' DNA sequence encoding a leader peptide that targets the protein for secretion in eukaryotic cells. An exemplary leader peptide sequence is given in SEQ ID NO:25. The synthesized gene was subcloned into the pcDNA3.4 plasmid using molecular biology reagents according to the manufacturer's instructions.
[0150] Use Expi293F cells (Thermo Fisher Scientific) to carry out the transfection of the plasmid, and the cells are cultivated at 37°C in a shaker ...
Embodiment 3
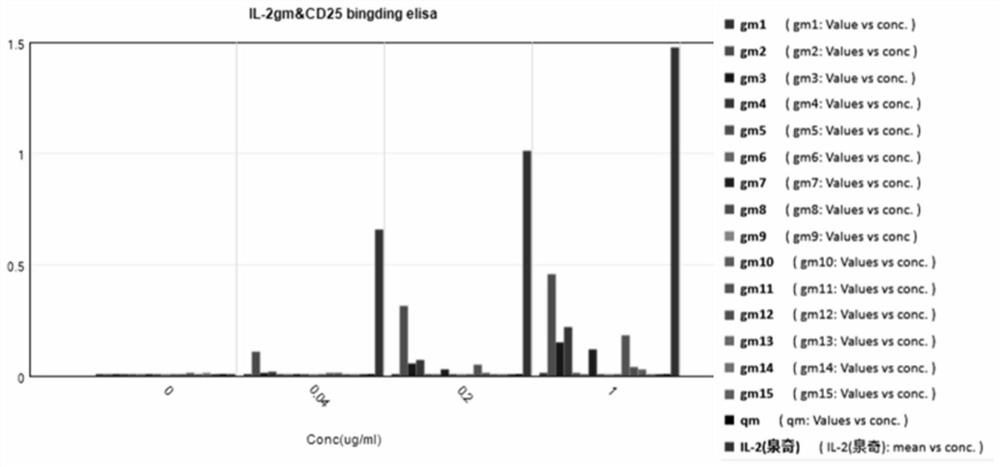
[0152] Example 3. Using ELISA, Fortebio or biacore to detect the binding affinity experiment of CD25
[0153] The present inventors detected the binding ability of IL-2 mutants to CD25 by enzyme-linked immunosorbent assay.
[0154] CD25 (derived from Example 2) was coated onto a 96-well high-adsorption microplate (3590, Costar), washed and blocked. Dilute the sample to be tested to an appropriate concentration and add it to the well. TMB color development, microplate reader (M5, Molerlder Devies), the wavelength is 450 / 650nm, read the signal value of each well. rhIL-2 is recombinant human interleukin-2 for injection (Quanqi).
[0155] Table 1. Summary of binding activity of rhIL-2 to IL-2 mutants and CD25
[0156]
[0157] Note: The binding activity of each concentration point of IL-2gm was compared with rhIL-2 (binding activity 100%).
[0158] The results are shown in figure 1 . It can be seen from the figure that at the experimental concentration, it can be clearly ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com