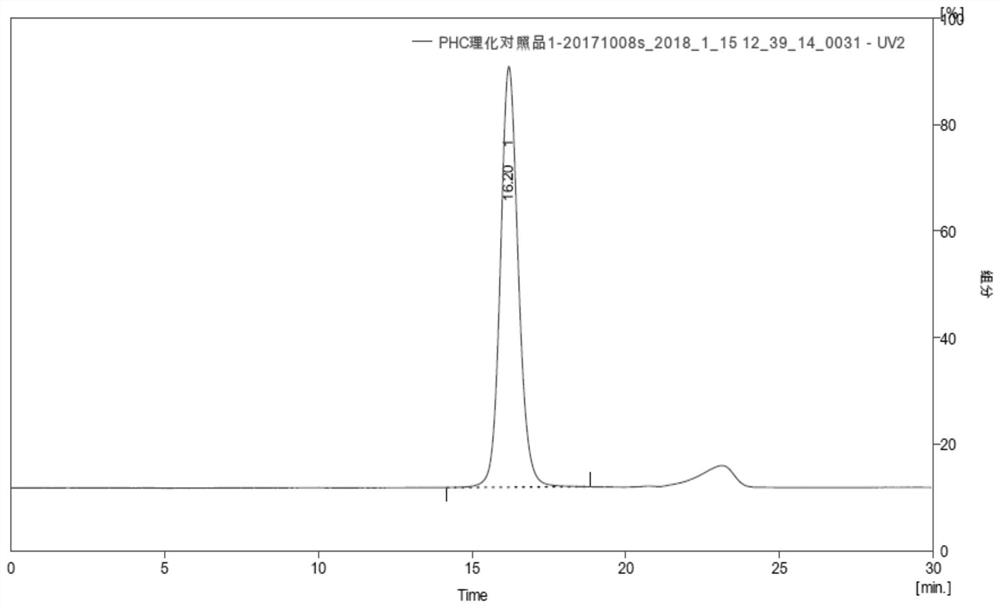
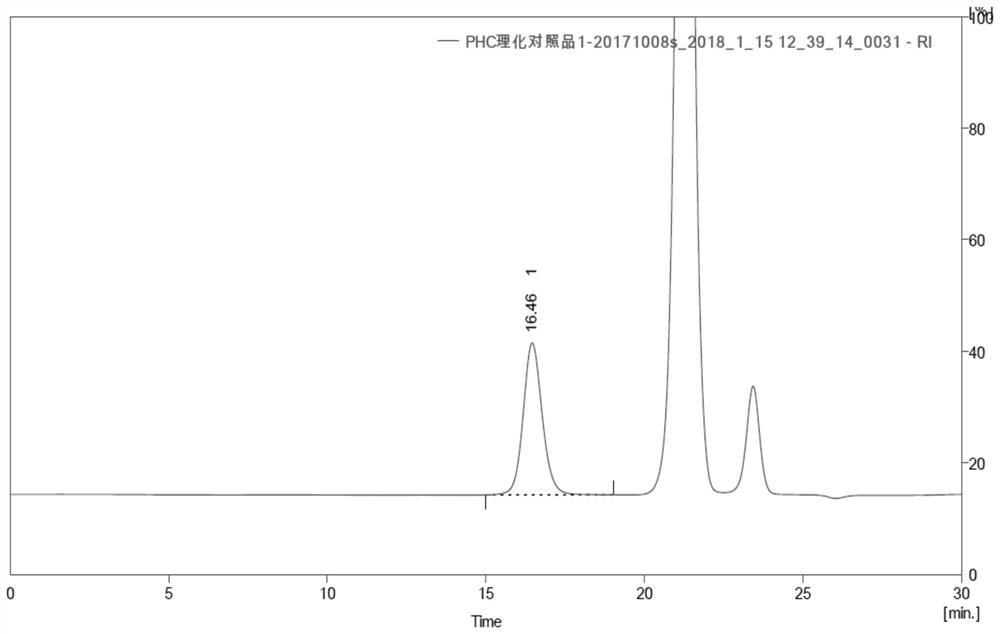
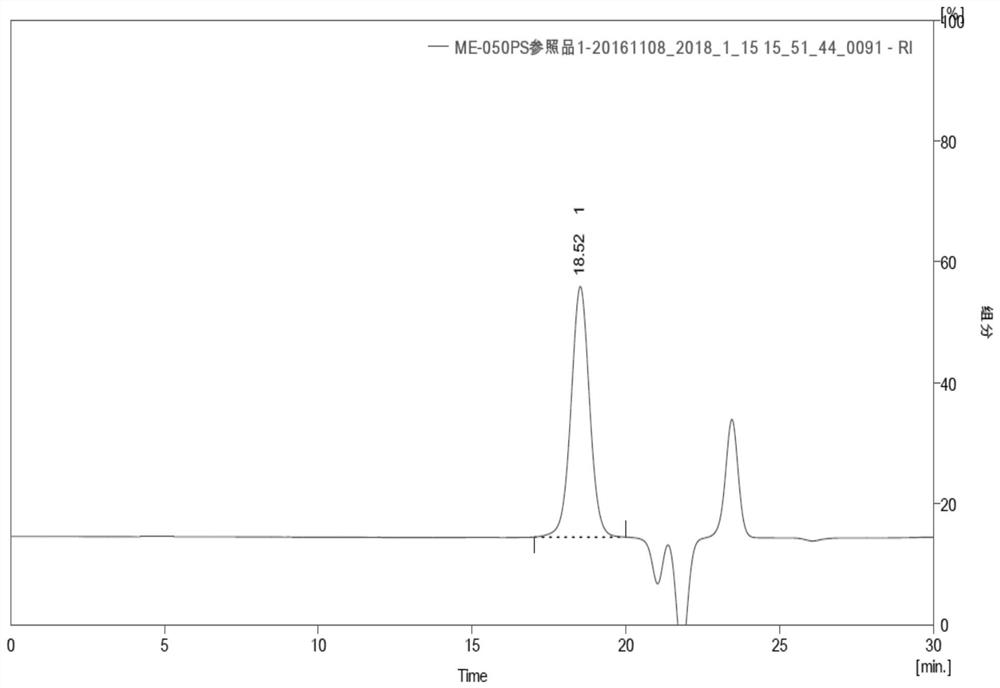
Urate oxidase modified by polyethylene glycol
A uric acid oxidase and polyethylene glycol technology, applied in the field of biomedicine, can solve problems such as unavailable long-term treatment, unresolved immunogenicity, and weakened efficacy of uricase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0109] In the preparation method of the polyethylene glycol-modified urate oxidase, various purification methods are used to obtain the high-purity polyethylene glycol-modified urate oxidase.
[0110] In another preferred embodiment, the purification of the modified sample includes but not limited to molecular sieve chromatography, ion exchange chromatography, hydrophobic chromatography, tangential flow ultrafiltration, or a combination. More preferred are molecular sieve chromatography and tangential flow ultrafiltration.
[0111] In another aspect of the present invention, the above polyethylene glycol-modified urate oxidase and its application are provided. The conjugate can achieve long-acting effect in vivo and significantly reduce the blood uric acid level, and can be used for the treatment of hyperuricemia and ventilation.
[0112] The polyethylene glycol urate oxidase is more suitable as a medicine for treating chronic hyperuricemia or ventilation and its composition....
Embodiment 1
[0131] The preparation of embodiment 1 recombinant uric acid oxidase
[0132] 1.1 Construction of genes and expression plasmids for uricase expression
[0133] According to the codon usage bias data of E.coli, combined with factors such as codon bias and GC content, the cDNA sequence of uricase protein (code name: PHC) (SEQ ID NO: 1) was designed, the whole gene was synthesized, and named as pUC-57-PHC plasmid. Nde I and BamH I were used as the insertion site of the target gene, and the pET-30a plasmid was used as the expression vector (pET-30a-PHC).
[0134] 1.2 Transformation of expression plasmids into bacterial host cells
[0135] Using CaCl 2 The expression vector pET-30a-PHC was introduced into Escherichia coli BL21(DE3) by the method, and Kanamycin was screened for resistance, and high-expression clones were screened out, and the original seed bank strain (E3B) was preserved. These steps are in accordance with the molecular biology field Common methods are implement...
Embodiment 2
[0140] Embodiment 2 Preparation of pegylated oxidative uricase
[0141] Dissolve 5KD molecular weight N-succinimide propionate PEG (5K-PEG-SPA) with 2mmol / L HCl into a 200mmol / L PEG solution. Enzyme: 5K-PEG-SPA), wherein the molar ratio of urate oxidase to 5K-PEG-SPA is calculated according to the monomer form, that is, the molar ratio of 1:55 to 1:95 refers to the monomer form of urate oxidase and 5K -The molar ratio of PEG-SPA is added to the carbonate buffer solution with a carbonate concentration of 0.1-0.3 mol / L and a pH of 10.0 dissolved in urate oxidase, so that the coupling reaction between PEG and urate oxidase occurs, and the coupling reaction Stir the reaction at 5-30°C for more than 60 minutes until the degree of PEG coupling no longer changes with time. After the reaction is complete, unmodified PEG and by-products are removed from the reaction by ultrafiltration and / or chromatography. A suitable molecular sieve chromatography medium can be selected to separate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com