Agkistrodon halas hemocoagulase characteristic polypeptide and application thereof in species identification of hemocoagulase for injection
A technology for hemagglutinin and injection, which is applied in the direction of enzymes, peptidases, hydrolytic enzymes, etc., can solve the problems that the source of unknown samples cannot be confirmed and the source of biochemical drugs can be identified, so as to improve the level of drug quality control and guarantee Safety and efficacy, strong specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of relevant reagent reagent and solution in the following examples is as follows:
[0033] (1) Reagents: trypsin (Sigma, lot number SLBS8956), haemagglutination of Agkistrodon venom (Jinzhou Aohong Pharmaceutical Co., Ltd., purity 98.5%), guanidine hydrochloride (VETEC, lot number WXBC4261V), trihydroxyaminomethane (Shanghai Stock Exchange , batch number 20181206), dithiothreitol (BBI Life Sciences, batch number D911BA0011), iodoacetamide (BBI Life Sciences, batch number B326BA1943), and other reagents were of analytical grade.
[0034] (2) 25 mmol / L ammonium bicarbonate solution: Weigh 79.06 mg of ammonium bicarbonate, add 40 mL of water to dissolve, and obtain.
[0035] (3) 0.4 mol / L dithiothreitol (DTT) solution: Weigh 15.42 mg of dithiothreitol, add 250 μL of water to dissolve, and get ready.
[0036] (4) 0.4 mol / L iodoacetamide solution (newly prepared for immediate use): Weigh 18.5 mg iodoacetamide, add 250 μL water to dissolve, and get rea...
Embodiment 1
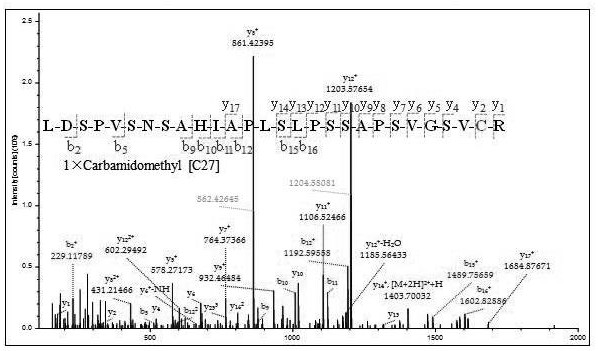
[0038] Example 1 Screening and determination of characteristic polypeptides.
[0039] 1. Instruments and equipment
[0040] Thermo Fusion high-resolution mass spectrometer (Thermo Fisher Scientific, USA), EASY-nLC 1000 nanoliter liquid chromatograph (Thermo Fisher Scientific, USA), CP225D electronic balance (Sartorius, Germany), Sigma 3-30 K refrigerated centrifuge ( Sigma, Germany), Milli-QAdvantage A10 ultrapure water meter (Millipore, USA).
[0041] 2. Chromatography and mass spectrometry conditions
[0042] Chromatographic conditions: Chromatographic column: 0.2 mm × 3.5 cm (5 μm particle size) ReproSil-Pur C18-AQ Trap column made in the laboratory for desalting and enrichment, using 75 μm × 25 cm (3 μm particle size) ReproSil made in the laboratory -PurC18-AQ nanoliter analytical column separation. Mobile phase A was 2% acetonitrile in 0.1% formic acid in water; mobile phase B was 98% acetonitrile in 0.1% formic acid in water. The flow rate of the nanoliter separation...
Embodiment 2
[0050] 1. Instruments and equipment
[0051] SCIEX Triple Quad 6500 triple quadrupole mass spectrometer, CP225D electronic balance (Sartorius, Germany), Sigma 3-30 K refrigerated centrifuge (Sigma, Germany), Milli-QAdvantage A10 ultrapure water instrument (Millipore, USA).
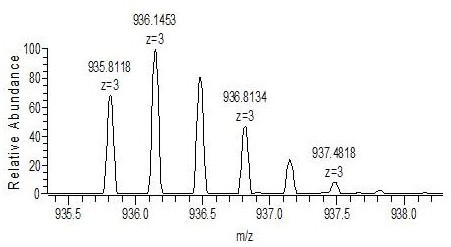
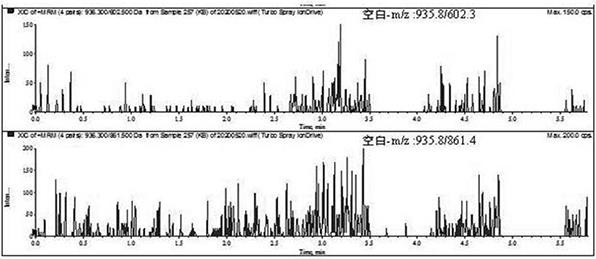
[0052] 2. Chromatography and mass spectrometry conditions
[0053] Liquid conditions: the chromatographic column is Waters ACQUITY UPLC BEH C 18 Chromatographic column (50mm×2.1 mm, 1.7μm); mobile phase A: 0.1% formic acid solution mobile phase, B: 0.1% formic acid acetonitrile; gradient elution, elution program: 0→1 min, mobile phase A 80%; 1→5 min, mobile phase A 80%→10%; 5→7 min, mobile phase A 10%→10%; column temperature: 40°C; injection volume: 2 μL; flow rate: 0.2 mL / min.
[0054] Mass spectrometry conditions: Electrospray ion source, positive ion scanning mode, multiple reaction monitoring; vortex ion spray temperature 500°C; ionization voltage 5.5 kV; collision cell outlet voltage 10 V; inlet vol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com