Intermediate isomer for preparing cefovecin by taking penicillin potassium salt as raw material and preparation method of intermediate isomer
A technology of penicillin potassium salt and cefvecin, applied in organic chemistry and other directions, can solve the problems of harsh reaction conditions, high environmental protection requirements, and difficult operation, and achieve the effects of good product quality, improved quality, and good reaction selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
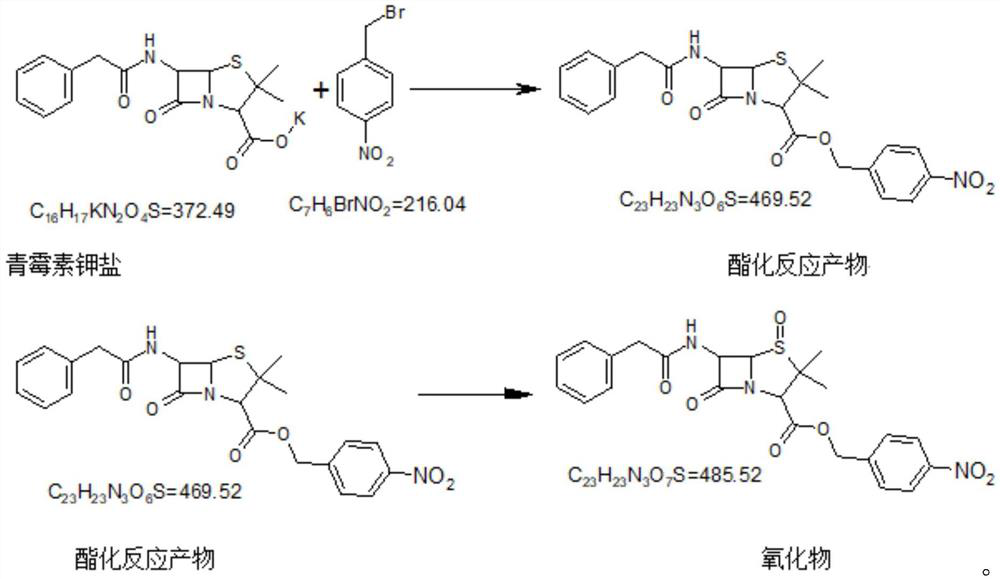
Embodiment 1
[0045] Esterification, Oxidation
[0046]
[0047] Add 200kg DMF and 69.6kg p-nitrobenzyl bromide into a 300L glass-lined reactor, stir at room temperature to dissolve and clarify the feed solution. Add 100kg (0.2685mol) penicillin potassium salt; raise the temperature to 42-45°C; stir and react at 42-45°C for 2h until the reaction is complete.
[0048] Add the reaction solution to 400kg of dichloromethane and 350kg of deionized water cooled to -5-0°C, stir, then add sulfuric acid solution to adjust the pH to less than 5, stir, separate the organic layer, and discard the water layer.
[0049] Cool the organic layer to about 0-5°C, add about 102kg of 32% peroxyacetic acid (freshly prepared) dropwise, and stir for about 2 hours until the reaction is complete. The layer was discarded, and the organic layer was added with about 28kg10% sodium sulfite aqueous solution until there was no oxidation (starch potassium iodide test paper does not change color) in the material, stirri...
Embodiment 2
[0051] Esterification, Oxidation
[0052]Add 200kg DMF and 62kg p-nitrobenzyl bromide into a 300L glass-lined reactor, stir at room temperature to dissolve and clarify the feed solution. Add 100kg (0.2685mol) penicillin potassium salt; raise the temperature to 42-45°C; stir the reaction at 42-45°C until the reaction is complete.
[0053] Add the reaction solution to 400kg of dichloromethane and 350kg of deionized water cooled to -5-0°C, stir, then add sulfuric acid solution to adjust the pH to less than 5, stir, separate the organic layer, and discard the water layer.
[0054] Cool the organic layer to about 0-5°C, add about 130kg of 32% peroxyacetic acid (freshly prepared) dropwise, stir the reaction until the reaction is complete after the dropwise addition, add 380kg of deionized water, stir, then let the layers stand, discard the water layer Go, organic layer is in adding about 30kg10% sodium sulfite aqueous solution, until there is no oxidation in the material (starch po...
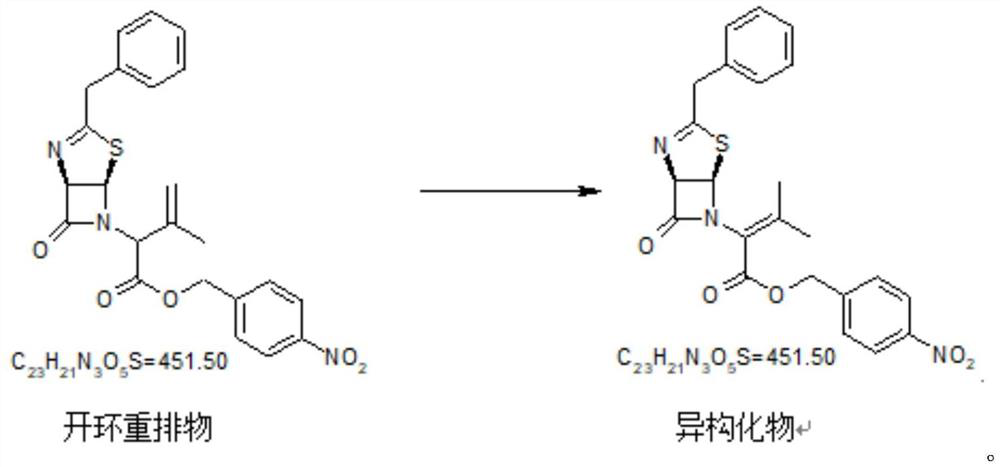
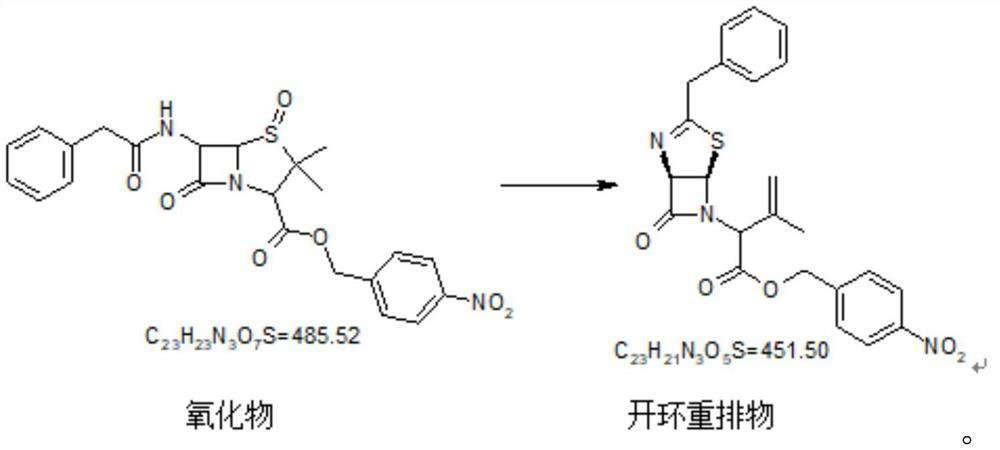
Embodiment 3
[0056] Ring-opening rearrangements and isomerizations
[0057]
[0058] Add 460kg of dehydrated toluene to the reaction kettle, add 100kg of intermediate oxides, wash the feeding port with 20kg of toluene, adjust the internal temperature to 90-100°C, stir until dissolved and clear, add 51.1kg of TMP, adjust the vacuum to control the internal temperature at 90°C -100°C, react until the reaction is complete. Nitrogen protection cools to below 30°C.
[0059] Add 27kgTEA under the protection of nitrogen, and react in the dark below 30°C (generally, the reaction is at room temperature, when the room temperature is higher than 30°C, properly lower the temperature) until the reaction is complete, add 200kg of 5% (W) hydrochloric acid, and stir for 5 minutes. The layers were allowed to stand, and the water layer was transferred to an extraction kettle. 250 kg of dichloromethane was added to the water layer and stirred for 5 minutes. Stand to separate layers, discard the water lay...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com