Crizotinib prodrug polymeric micelle co-loaded with chemotherapeutic drug and preparation method thereof
A technology for crizotinib and chemotherapeutic drugs, which is applied in the field of polymer materials and new dosage forms of pharmaceutical preparations, can solve the problems of destroying the micellar structure, residual active groups, side effects, etc., achieves efficient co-loading, and improves immunogenicity. , the effect of synergistic anti-tumor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
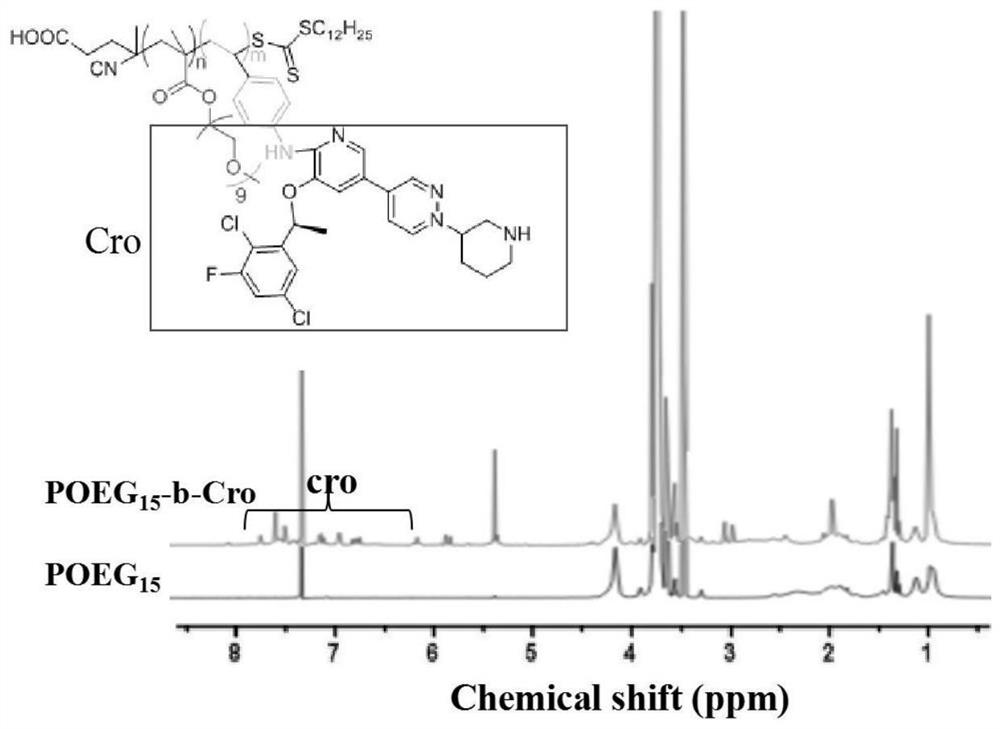
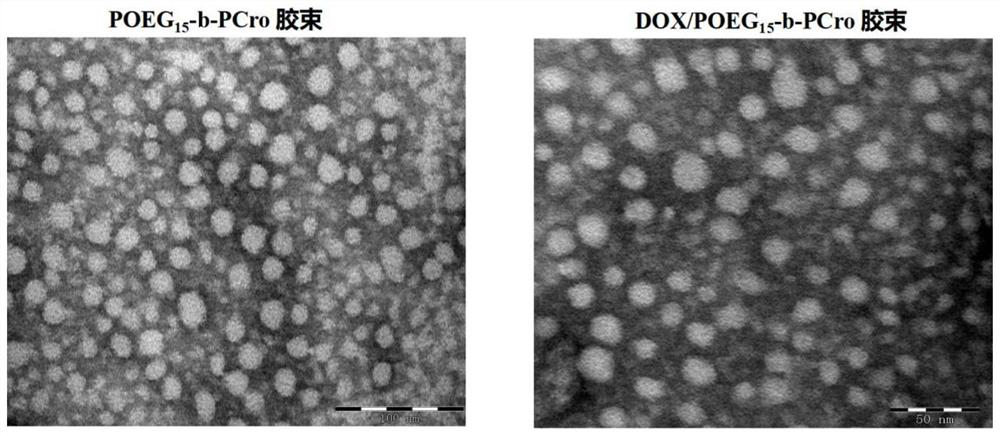
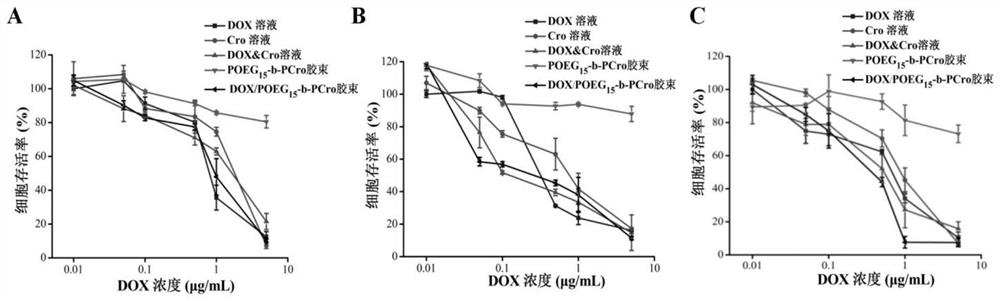
[0030] The invention relates to a crizotinib prodrug polymer micelle co-loaded with chemotherapeutic drugs and a preparation method thereof. The present invention is based on polymer micelles combined with prodrug technology, and polymerizes POEG monomer and crizotinib monomer, a tyrosine kinase inhibitor drug, into a hydrophilic diblock copolymer through RAFT polymerization method, and further physically entraps other chemotherapeutic agents. The drug self-assembles into prodrug polymer micelles. The invention can polymerize crizotinib prodrug polymers with different molecular weights by effectively regulating the number of POEG monomers and crizotinib monomers, so as to increase the effective loading capacity of chemotherapy drugs. Under the long cycle mediated by EPR, the co-drug-loaded micelles can be precisely and effectively co-accumulated in tumor tissue. After endocytosis, the co-drug-loaded micelles disintegrated and released the two drugs. The released crizotinib pr...
Embodiment 1
[0054] Crizotinib Prodrug Polymer POEG 15 -b-Cro 9 Synthesis.
[0055] Synthesis of crizotinib monomer.
[0056] 0.2 g of crizotinib, 0.068 g of chloromethylstyrene and 0.14 g of anhydrous sodium carbonate were co-dissolved in 5.5 mL of DMF, and reacted with magnetic stirring in an oil bath at 50°C for 4 h. After the reaction was completed, after three extractions with ethyl acetate and water, the filtrate was collected and rotary evaporated, with dichloromethane and methanol (10:1, v / v) as the mobile phase, dichloromethane and methanol (10:1, v / v) is the developer, and the product is purified by gradient elution of silica gel column chromatography. The purified product is collected and rotary evaporated, and vacuum-dried at room temperature to obtain a pale yellow oily crizotinib monomer.
[0057] Synthesis of POEG monomers.
[0058] Add 1.52g of OEGMA, 60mg of 4-cyano-4-(thiobenzoyl)valeric acid and 5mg of azobisisobutyronitrile into a dry eggplant-shaped reaction flask,...
Embodiment 2
[0062] Crizotinib Prodrug Polymer POEG 10 -b-PCro 6 Synthesis.
[0063] (1) Synthesis of crizotinib monomer.
[0064] 0.2 g of crizotinib, 0.068 g of chloromethylstyrene and 0.14 g of anhydrous sodium carbonate were co-dissolved in 5.5 mL of DMF, and reacted with magnetic stirring in an oil bath at 50°C for 4 h. After the reaction was completed, after three extractions with ethyl acetate and water, the filtrate was collected and rotary evaporated, with dichloromethane and methanol (10:1, v / v) as the mobile phase, dichloromethane and methanol (10:1 , v / v) as the developer, the purified product was eluted by gradient elution of silica gel column chromatography, collected and rotary evaporated the purified product, and vacuum-dried at room temperature to obtain light yellow oily crizotinib monomer.
[0065] Synthesis of POEG monomers.
[0066] Add 1.52g of OEGMA, 60mg of 4-cyano-4-(thiobenzoyl)valeric acid and 5mg of azobisisobutyronitrile into a dry eggplant-shaped reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com