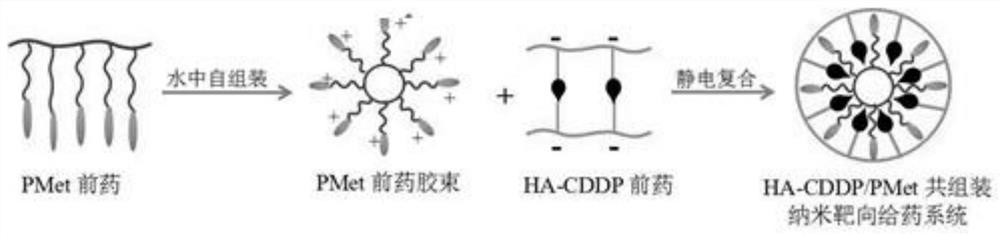
A double prodrug co-assembled nano-targeted drug delivery system and its preparation method
A technology of targeted drug delivery and co-assembly, applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve problems such as large differences in physical and chemical properties, incompatibility, etc., to achieve efficient co-loading and improve synergistic anti-tumor efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Synthesis of hyaluronic acid-cisplatin (HA-CDDP) polymer prodrug
[0043] Dissolve 30mg of cisplatin and 33mg of silver nitrate in 5mL of distilled water, protect from light, stir magnetically in a water bath at 50°C for 3h, then react magnetically for 24h at room temperature, centrifuge the resulting reaction solution at 1000rpm for 5min, filter to remove silver chloride, and obtain cisplatin intermediate body;
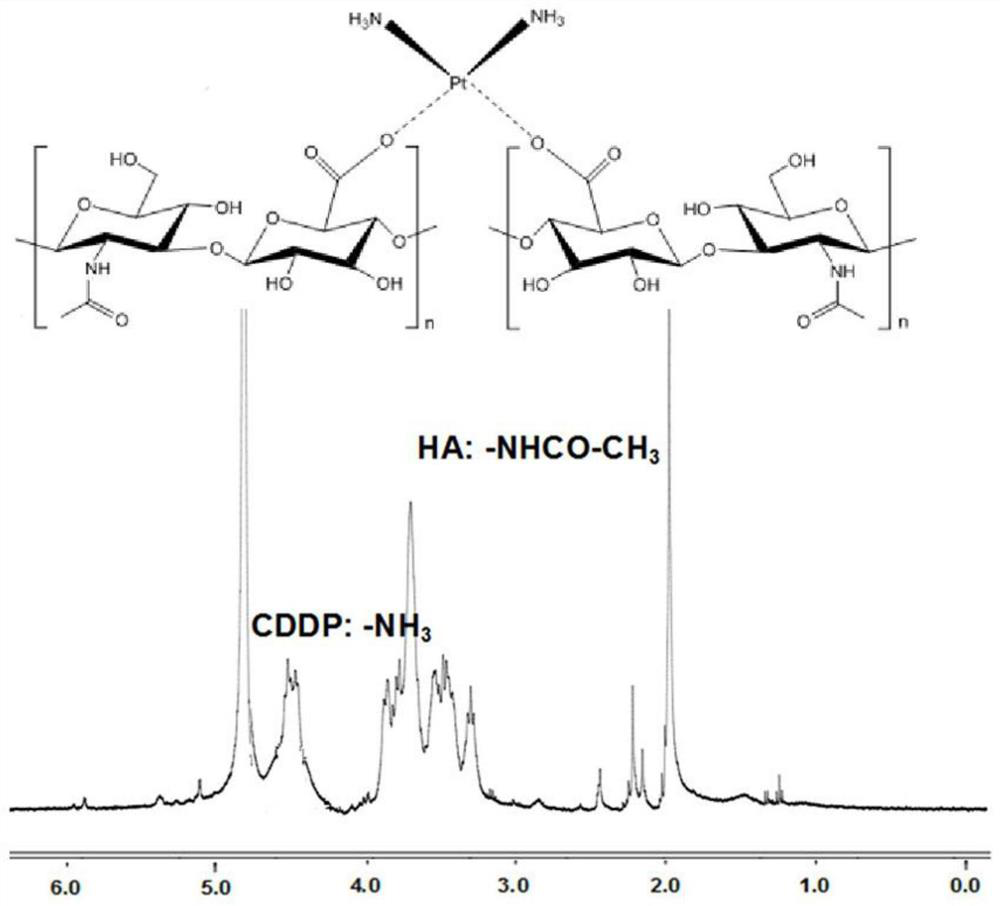
[0044] Dissolve 200mg of hyaluronic acid in 4mL of distilled water and add it to the cisplatin intermediate solution obtained above, avoid light, and react with magnetic stirring at room temperature for 24 hours. CDDP polymer prodrugs. That 1 H NMR spectrum such as figure 1 shown.
[0045] 2. Synthesis of polystyrene-metformin (PMet) prodrug
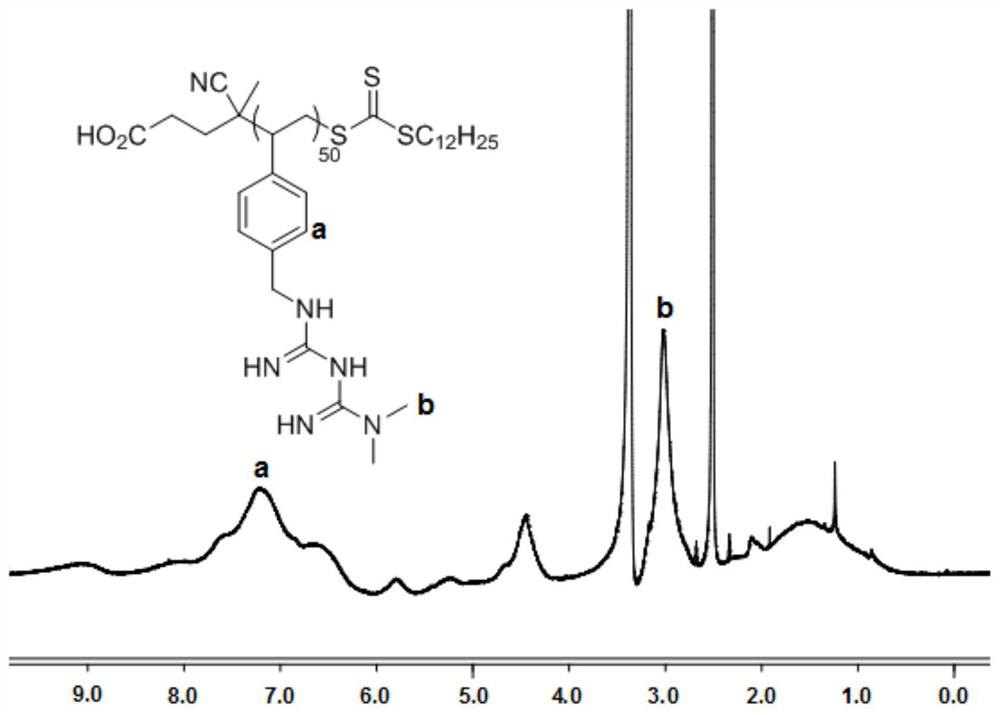
[0046] Add 1.5 g of 4-styrene chloride, 10 mg of 4-cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]valeric acid and 1 mg of azobisisobutyronitrile into a dry reaction flask, Dissolve in 2mL of anhydrous tetrahydr...
Embodiment 2
[0052] 1. Synthesis of hyaluronic acid-cisplatin (HA-CDDP) polymer prodrug
[0053] Dissolve 40mg of cisplatin and 50mg of silver nitrate in 8mL of distilled water, protect from light, stir magnetically in a water bath at 70°C for 6h, then react magnetically for 20h at room temperature, centrifuge the resulting reaction solution at 1000rpm for 10min, filter to remove silver chloride, and obtain cisplatin intermediate body;
[0054] Dissolve 400mg of hyaluronic acid in 10mL of distilled water and add it to the cisplatin intermediate solution obtained above, keep away from light, and react with magnetic stirring at room temperature for 48h; CDDP polymer prodrugs. That 1 H NMR spectrum such as figure 1 shown.
[0055] 2. Synthesis of polystyrene-metformin (PMet) prodrug
[0056] Add 1g of 4-styrene chloride, 15mg of 4-cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]valeric acid and 2mg of azobisisobutyronitrile into a dry reaction flask, dissolve In 6mL of anhydrous tetrahyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com