gRNA sequence of targeted KrasG12D mutant transcript, vector and application of vector
A transcript and sequence technology, applied in the field of medical biology, can solve problems such as poor treatment effect, and achieve the effect of significant technological progress, high safety, and improved targeting and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
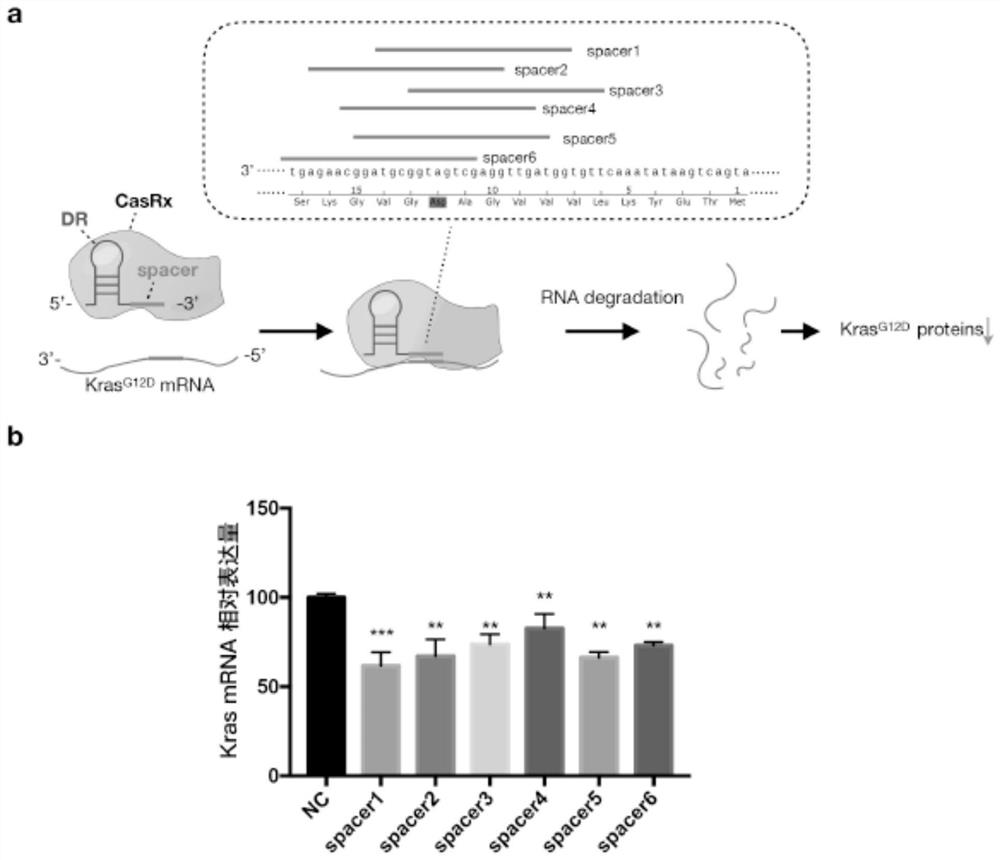
[0019] Example 1 Targeting Kras G12D Design of the spacer sequence of the gRNA of the transcript
[0020] Targeting Kras G12D A series of spacer sequences of the gRNA of the transcript are designed according to the following conditions:
[0021] 1) The length of the Spacer sequence is 22bp;
[0022] 2) Its 22bp sequence is complementary to the Kras mRNA sequence; (Kras mRNA see NCBI ReferenceSequence: NM_004985.5)
[0023] 3) The Spacer sequence must cover the single nucleotide mutation site A>G of Kras' G12D;
[0024] 4) One base and / or the last base at the 3' end of the Spacer is c.
[0025] According to the above four conditions, the designed spacer sequence is as follows:
[0026] 1) cctacgccatcagctccaacta; as shown in SEQ ID NO.1.
[0027] 2) ccatcagctccaactaccacaa; as shown in SEQ ID NO.2.
[0028] 3) cgccatcagctccaactaccac; as shown in SEQ ID NO.3.
[0029] 4) cttgcctacgccatcagctcca; as shown in SEQ ID NO.4.
[0030] 5) ctcttgcctacgccatcagctc; as shown in SEQ I...
Embodiment 2
[0032] Embodiment 2 contains targeting Kras G12D Construction and Characterization of Transcript-gRNA Adenoviruses
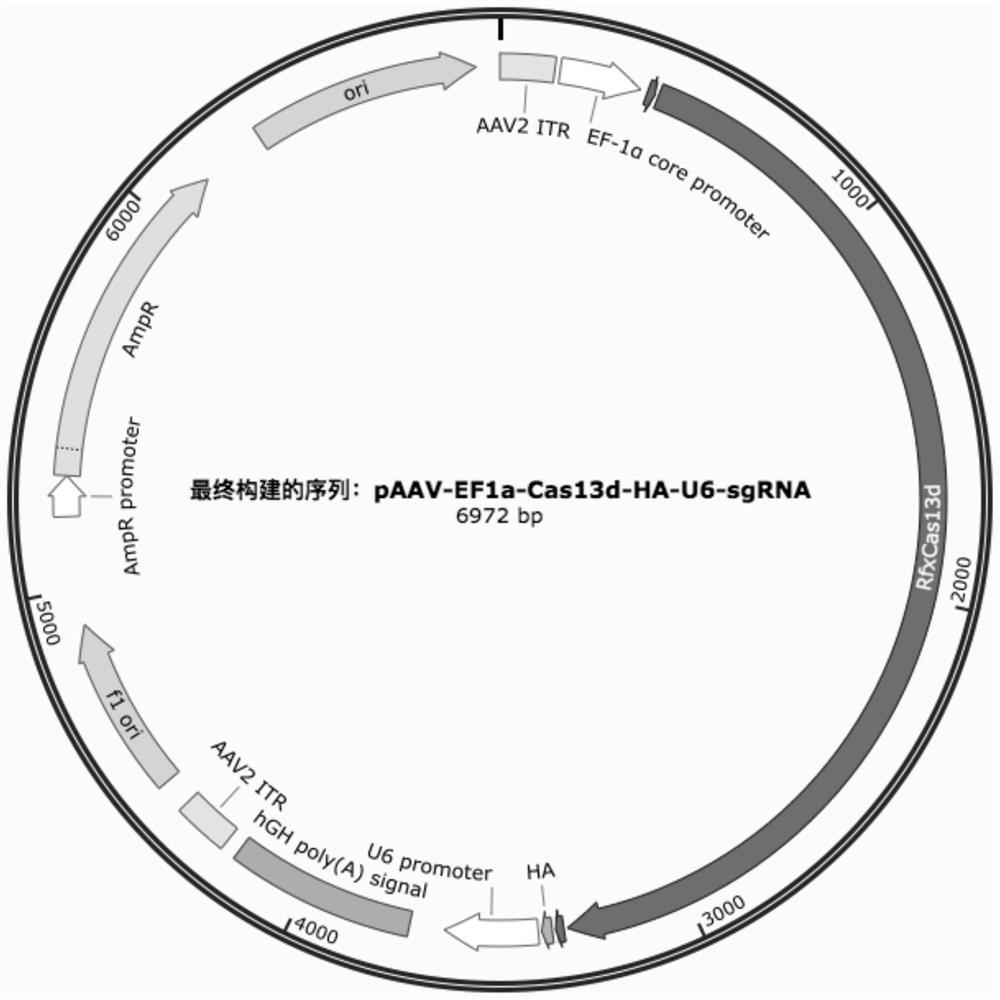
[0033] Such as figure 1 As shown, containing AmpR promoter, AmpR, ori, AAV2 ITR, EF-1α core promoter, SV40-NLS, RfxCas13d, SV40-NLS, HA, hU6 promoter, gRNA-DR, gRNA(DR+spacer), hGHpolyAsignal, AAV2 ITR, The adeno-associated virus vector backbone of f1 ori pAAV-EF1a-Cas13d-HA-U6-sgRNA, this adeno-associated virus vector backbone can carry different therapeutic gRNAs and is widely used in the field of gene therapy.
[0034] The successfully constructed vector is: pAAV-EF1a-Cas13d-HA-U6-sgRNA.
[0035]Wherein, the above spacer sequence: (1.cctacgccatcagctccaacta; 2.ccatcagctccaactaccaa; 3.cgccatcagctccaactaccac; 4.Cttgcctacgccatcagctcca; 5.ctcttgcctacgccatcagctc; 6.cactcttgcctacgccatcagc) is inserted through the site of BbsI.
[0036] BbsI (NEB) digestion AAV2 adeno-associated virus vector backbone vector: BbsI 1ul, 10×NEB buffer 2ul, backbone vector 1ug, water ...
Embodiment 3
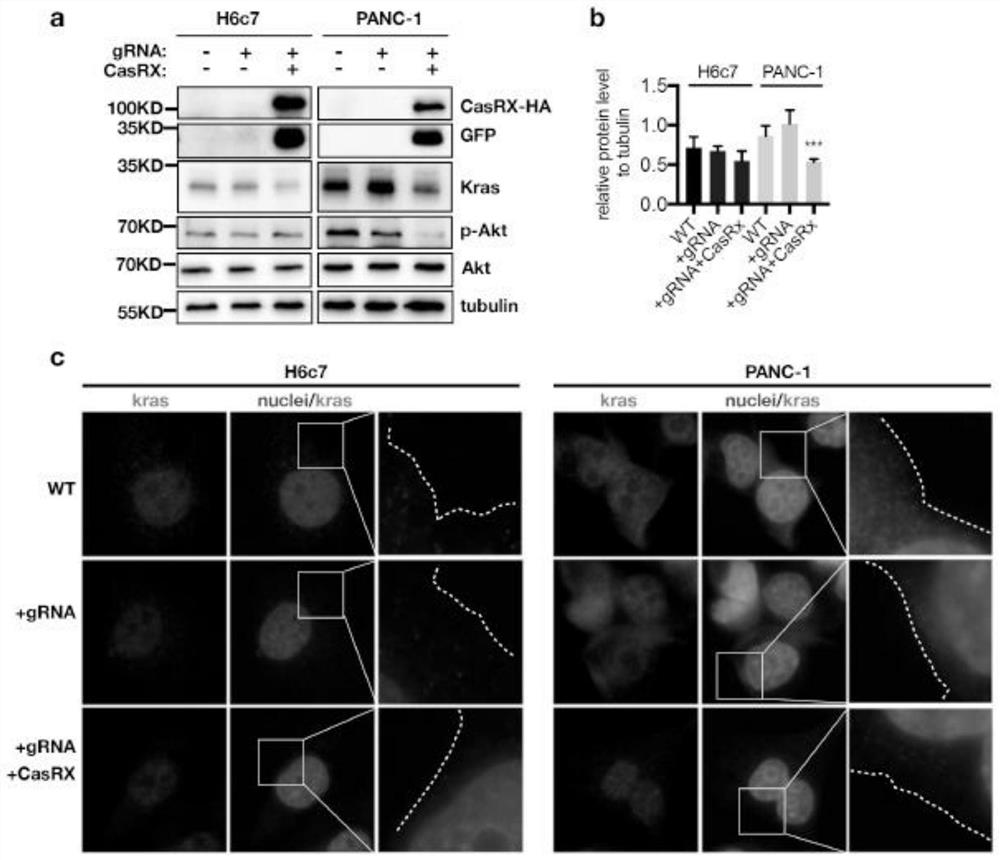
[0040] Example 3 Construction of AAV8 carrying CasRx-gRNA (titer about 1*10^13GC / ml).
[0041] The pAAV-EF1a-Cas13d-HA-U6-sgRNA plasmid was packaged with adeno-associated virus (AAV), and its titer was detected.
[0042] The specific method is as follows:
[0043] By using three AAV plasmids (pAAV8-rep / cap-Y-F mutant, pAAV-EF1a-Cas13d-HA-U6-sgRNA / pAAV2-GFP and pHelper ( pAAV2-GFP , pAAV8-rep / cap-Y-F mutant, pHelper three plasmids are commercially available products) transiently transfected 293T cells, resulting in recombinant AAV (rAAV) vector. 293T cells were transiently transfected with polyethyleneimine at 80% confluency. Cells were harvested 72 hours after transfection, lysed, and treated with 25 units / mL of benzoate nuclease. Subsequently, recombinant AAV was purified by iodixanol-based gradient density centrifugation followed by column chromatography. Recombinant AAV vectors were then concentrated to a final volume of 0.5 ml in phosphate buffered saline using Amicon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com