Capsule for treating ulcerative colitis
A capsule and ulcerative technology, applied in the field of capsules for the treatment of ulcerative colitis, can solve problems such as increased absorption, side effects, and the onset of pulmonary hypertension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
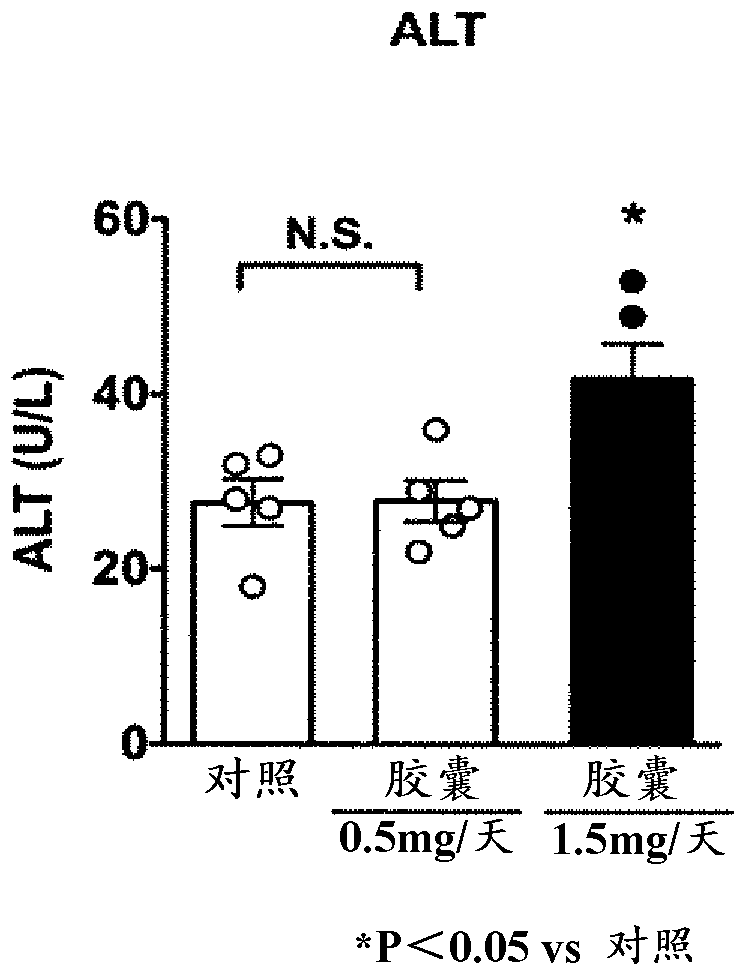
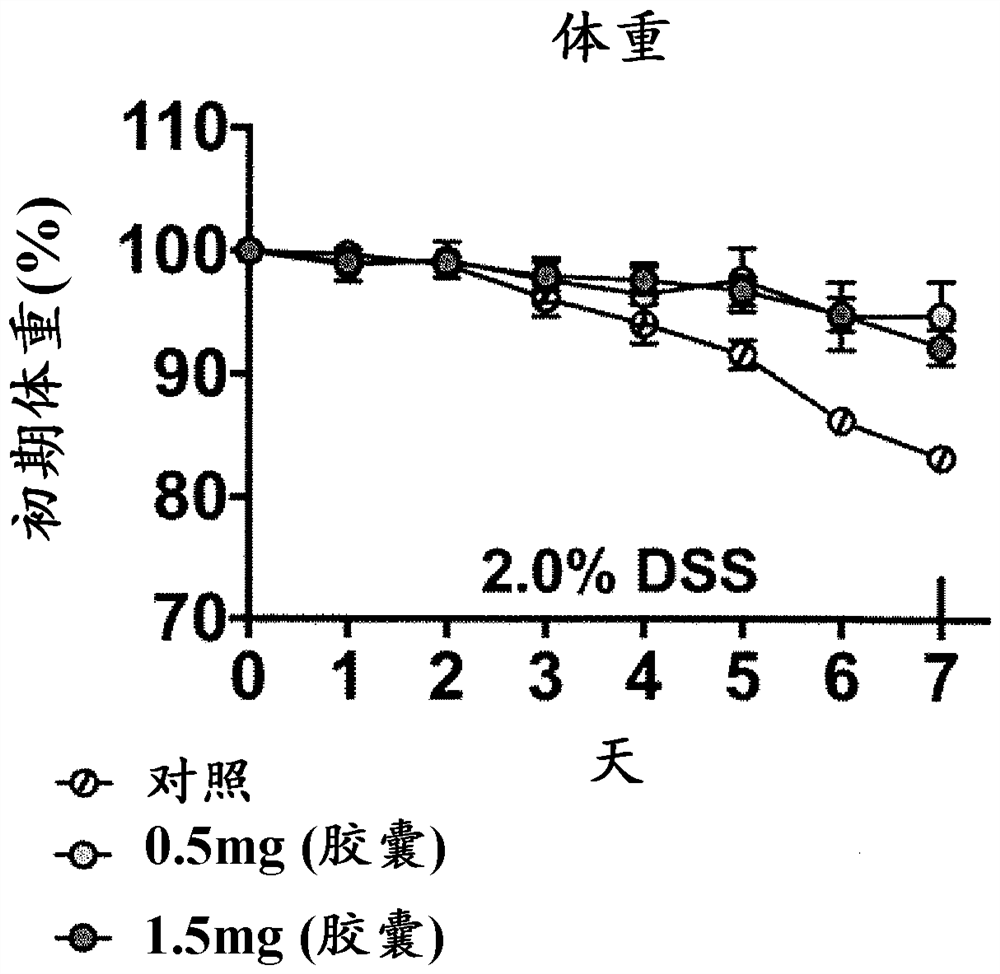
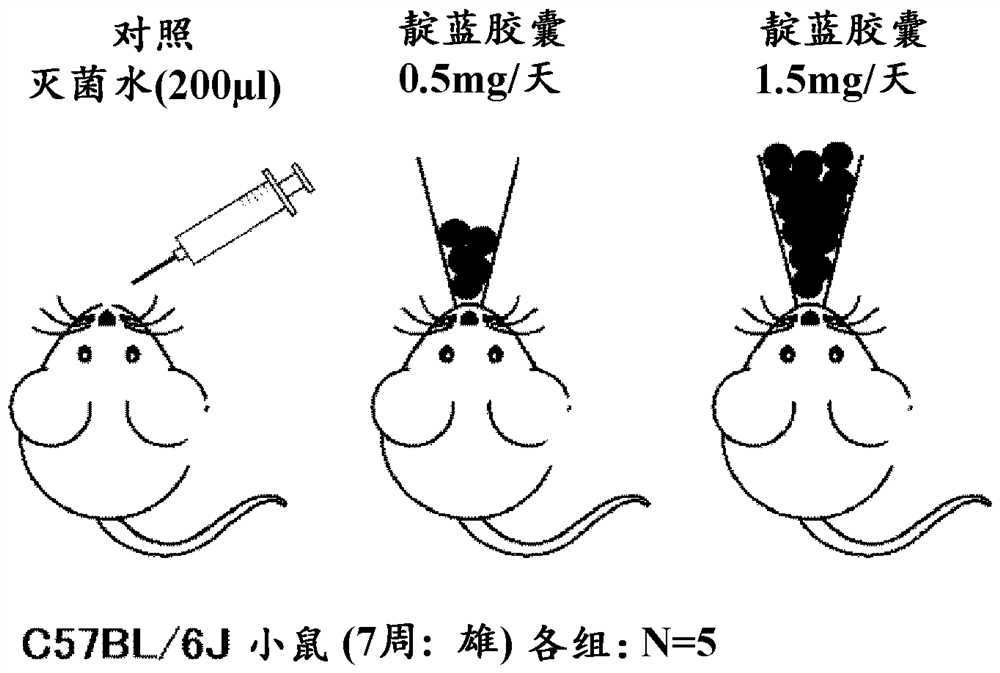
[0104] 5 capsules (corresponding to 0.5 mg of indigo) and 15 capsules (corresponding to 1.5 mg of indigo) of the above-mentioned indigo-blended capsule A were orally administered to 5 enteritis mouse samples (検body) for 7 days. It should be noted that in order to maintain the enteritis state of the mice for 7 days, the above-mentioned 2.0% DDS (dextran sulfate sodium salt) solution was also orally administered when the capsule A was orally administered.
[0105] Evaluation results
[0106] Regarding the enteritis inhibitory effect of indigo, according to the reduction of body weight (BW), enteritis activity score (Disease Activity Index Score (Disease Activity Index Score, DAI) score: the frequency of defecation, bloody stool, mucosal findings, overall Each item of the evaluation was scored on a scale of 4 from 0 to 3, and the total score (0 to 12)) and intestinal length (colon length, colon length) were evaluated. As the condition of colitis progressed, body weight decreased...
Embodiment 2
[0120] In order to cope with the high pH caused by hypochlorhydria, the indigo-blended capsule A of Preparation Example 1 was subjected to an acid treatment before drying to produce a capsule, and a disintegration test was performed.
[0121] Capsules containing indigo dipped in an acidic solution for 3 minutes or longer were prepared, and dipped for 60 minutes at 37° C. for 60 minutes at pH 4.0, which is the pH in the hypothetical mouse stomach, to conduct a disintegration test. After 60 minutes, the capsule was taken out into a glass container (Schale), and a photograph was taken to confirm the state of the capsule. Disintegration of the capsule and outflow of indigo in the capsule were not observed.
Embodiment 3
[0123] The following indigo-blend capsules B were prepared in the same manner as the indigo-blend capsules A of Preparation Example 1.
[0124] (a) Content solution: Disperse 8.33 g of indigo (manufactured by Tokyo Chemical Industry Co., Ltd., purity: 97%) in 85.67 g of melted oily substance (glycerin fatty acid ester) at 32°C, 3.00 lecithin and 3.00 tocopherol In the melting liquid, the obtained solution was used as the content liquid.
[0125] (b) Protective layer solution: Same as indigo-blended capsule A.
[0126] (c) Film solution: same as indigo-blended capsule A.
[0127] In order to confirm the improvement of the adhesion to the large intestine, an in vitro test was carried out using the following two virtual plates of large intestine cells (both manufactured by Corning International Co., Ltd.).
[0128] (1) BioCoat TM Fibronectin 6-well plate (BioCoat TM Fibronectin 6・well plate) (coating ingredient: fibronectin)
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com