Nitrogen-boron co-doped graphene composite denitration sulfur-resistant catalyst and preparation method thereof
A graphene composite and co-doping technology, applied in chemical instruments and methods, physical/chemical process catalysts, separation methods, etc., to achieve the effects of high denitration and sulfur resistance and good sulfur resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
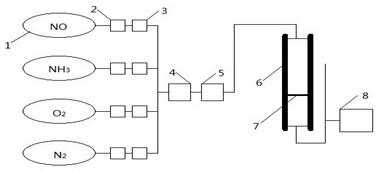
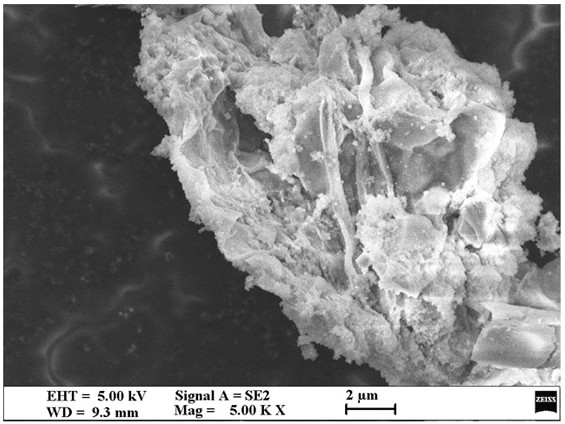
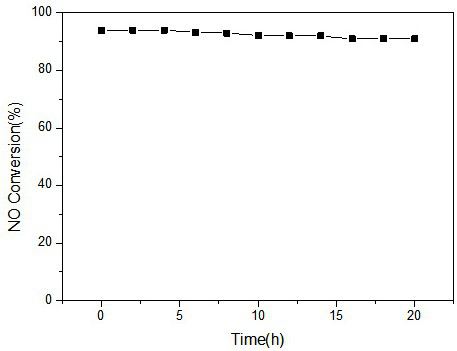
Image
Examples
Embodiment 1
[0027] Accurately weigh 0.1g of the above sample of self-made nitrogen-doped graphene oxide, dissolve it in 50mL of deionized water, add 0.06g of polyvinylpyrrolidone (referred to as PVP) after ultrasonication for 10min, and then dissolve 0.055g of cerium acetate ( Ce(Ac) for short 3 ) into the prepared above solution, put in a stirrer, and stir at room temperature for 1 hour until Ce(Ac) 3 completely dissolved. Then weigh 0.022g of cobalt chloride (CoCl 2 ), added to the above solution, and continued to stir at room temperature for 1 hour until CoCl 2 completely dissolved. Then accurately weigh 0.100gKMnO 4 Dissolve in 50mL of deionized water, and then add to the above reaction solution. Continue to react at room temperature for 1h, and weigh 0.1g of boric acid (abbreviated as H 3BO 3 ) and 0.1g sodium borohydride (referred to as NaBH 4 ) into the reaction solution, stirred until the boric acid was dissolved, transferred the reaction solution into a polytetrafluoroeth...
Embodiment 2
[0030] Accurately weigh 0.1g of the above sample of self-made nitrogen-doped graphene oxide, dissolve it in 50mL of deionized water, add 0.06g of polyvinylpyrrolidone (referred to as PVP) after ultrasonication for 10min, and then dissolve 0.065g of cerium acetate ( Ce(Ac) for short 3 ) into the prepared above solution, put in a stirrer, and stir at room temperature for 1 hour until Ce(Ac) 3 completely dissolved. Then weigh 0.027g of cobalt chloride (CoCl 2 ), added to the above solution, and continued to stir at room temperature for 1 hour until CoCl 2 completely dissolved. Then accurately weigh 0.199gKMnO 4 Dissolve in 50mL of deionized water, and then add to the above reaction solution. Continue to react at room temperature for 1h, and weigh 0.1g of boric acid (abbreviated as H 3 BO 3 ) and 0.1g sodium borohydride (referred to as NaBH 4 ) into the reaction solution, stirred until the boric acid was dissolved, transferred the reaction solution into a polytetrafluoroet...
Embodiment 3
[0033] Accurately weigh 0.1g of the above sample of self-made nitrogen-doped graphene oxide, dissolve it in 50mL of deionized water, add 0.06g of polyvinylpyrrolidone (referred to as PVP) after ultrasonication for 10min, and then dissolve 0.075g of cerium acetate ( Ce(Ac) for short 3 ) into the prepared above solution, put in a stirrer, and stir at room temperature for 1 hour until Ce(Ac) 3 completely dissolved. Then weigh 0.031g of cobalt chloride (CoCl 2 ), added to the above solution, and continued to stir at room temperature for 1 hour until CoCl 2 completely dissolved. Then accurately weigh 0.299gKMnO 4 Dissolve in 50mL of deionized water, and then add to the above reaction solution. Continue to react at room temperature for 1h, and weigh 0.1g of boric acid (abbreviated as H 3 BO 3 ) and 0.1g sodium borohydride (referred to as NaBH 4 ) into the reaction solution, stirred until the boric acid was dissolved, transferred the reaction solution into a polytetrafluoroet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com