Quinoline compound or pharmaceutically acceptable salt thereof for treating giant-cell tumor of bone
A technology for giant cell tumor of bone and a compound, which is applied in the field of quinoline compounds or their pharmaceutically acceptable salts, can solve the problems of slow progress of chemotherapy, incomplete curettage, increased difficulty in excision, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
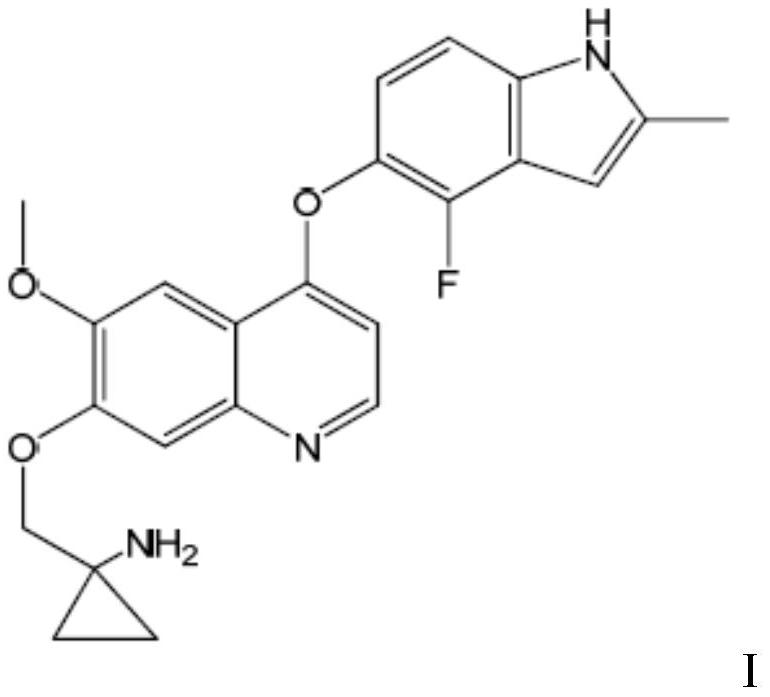
[0068] Example 1 1-[[[4-(4-fluoro-2-methyl-1H-indol-5-yl)oxy-6-methoxyquinolin-7-yl]oxy]methyl] Cyclopropylamine dihydrochloride
[0069]
[0070]1-[[[4-(4-fluoro-2-methyl-1H-indol-5-yl)oxy-6-methoxyquinolin-7-yl) was prepared according to the method of Example 24 in WO2008112407 ]oxyl]methyl]cyclopropylamine, and then refer to the preparation method of "Example of Salt Form" in the specification of WO2008112407 to prepare the title compound.
[0071] Or it can be prepared by referring to the method disclosed in Chinese patent application CN102344438A.
Embodiment 21
[0072] Example 2 1-[[[4-(4-fluoro-2-methyl-1H-indol-5-yl)oxy-6-methoxyquinolin-7-yl]oxy]methyl] Preparation of capsules of cyclopropylamine dihydrochloride (dihydrochloride of compound I)
[0073]
[0074] Grind the dihydrochloride of compound I and pass through a 80-mesh sieve; then mix evenly with mannitol and hydroxypropyl cellulose; then add the prescribed amount of microcrystalline cellulose, mix evenly, and pass through a 0.8mm sieve; finally add the prescribed amount The magnesium stearate is mixed well and filled into capsules.
[0075] Capsules with other contents of the dihydrochloride of Compound I can be prepared with reference to the same ratio and prescription as above.
Embodiment 3
[0076] Embodiment 3 in vitro experiments
[0077] Test drug:
[0078] 1-[[[4-(4-fluoro-2-methyl-1H-indol-5-yl)oxy-6-methoxyquinolin-7-yl]oxy]methyl]cyclopropylamine di Hydrochloride (referred to as the dihydrochloride of compound I); vincristine; doxorubicin; cyclophosphamide.
[0079] 1. The effect of dihydrochloride of compound I on inhibiting the growth of giant cell tumor of bone
[0080] Preparation method:
[0081] The dihydrochloride of compound I was made into the required concentration with distilled water;
[0082] Experimental animal preparation:
[0083] BALB / cA-nude nude mice, feeding environment: SPF grade. Nude mice were subcutaneously inoculated with human giant cell tumor cells of bone (source of giant cell tumor of bone: primary human GCT cells isolated from tumor tissues of confirmed patients. For the isolation method of GCT stromal cells, refer to the method provided by Smink et al.), wait until the tumor grows to 100-250mm 3 Afterwards, the animals ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com