Application of compound in treatment of autoimmune skin diseases caused by inflammation
An autoimmune and compound technology, which is applied in the application field of compounds in the treatment of autoimmune skin diseases caused by inflammation, and can solve problems such as adverse reactions, large toxic and side effects, and poor curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] Embodiment 1 (treatment of psoriasis by oral Nib1):
[0176] 1. Experimental reagents:
[0177] 5% imiquimod ointment, Aldara, 3M Pharmaceuticals; dexamethasone ointment: Shanghai Sanjiu Pharmaceutical; PEG300, Sigma, product number 90878. Nib1(pimozide):Sigma(P1793).
[0178] 2. Experimental plan:
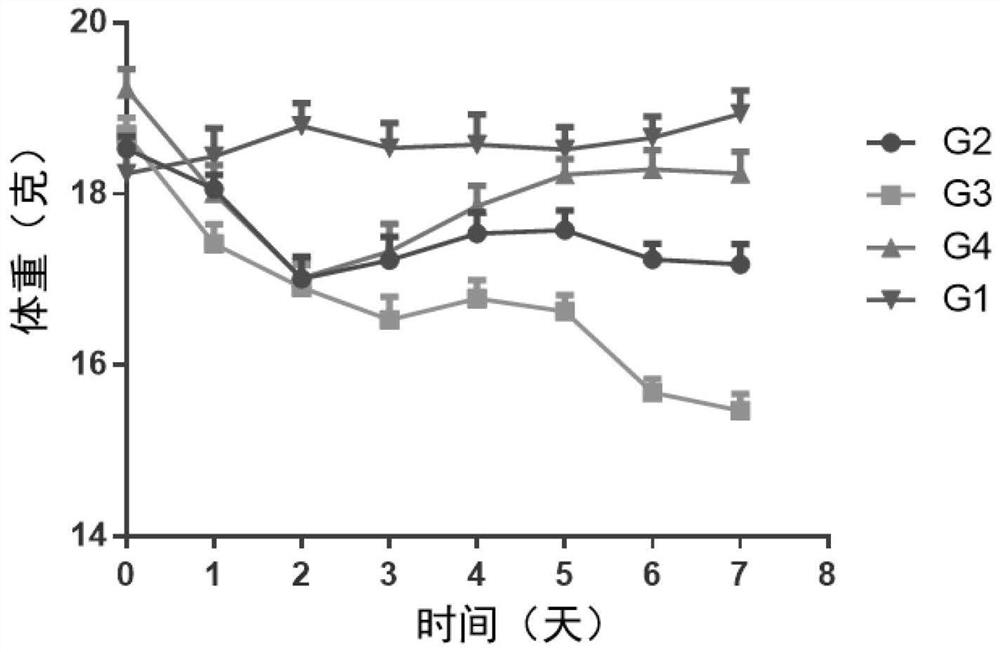
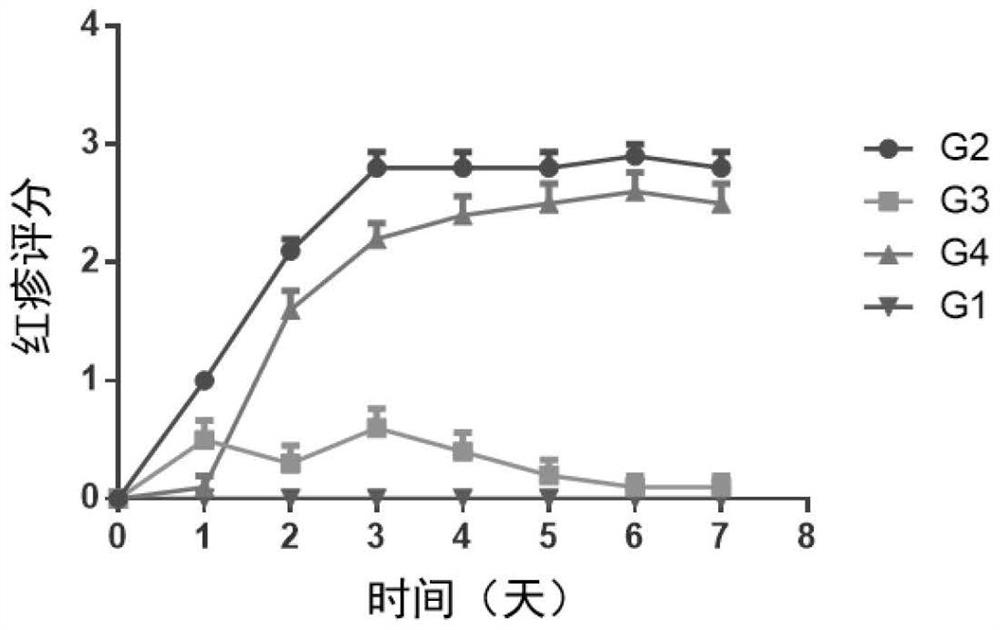
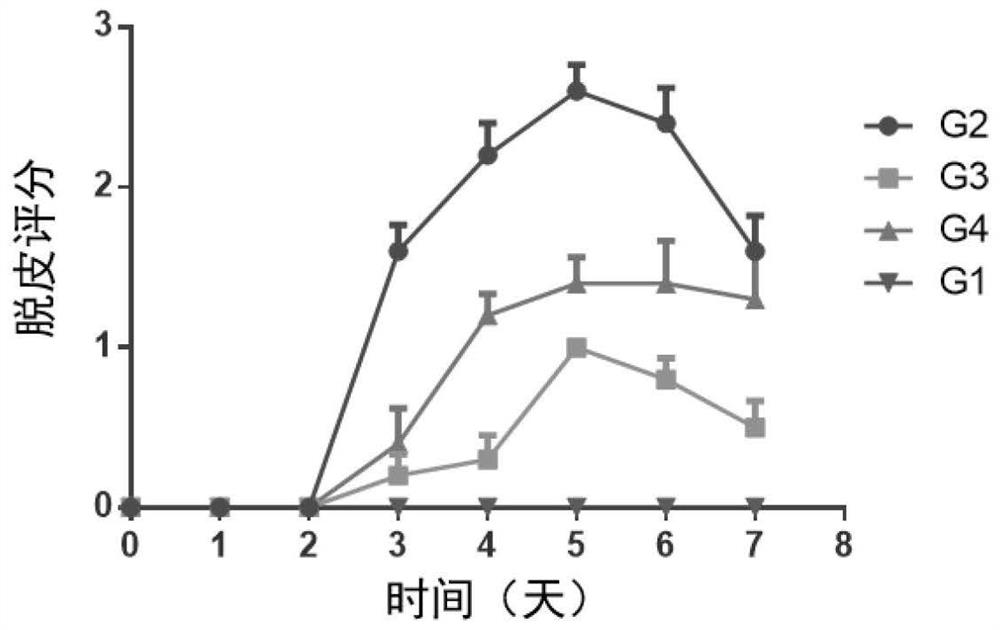
[0179] Female BALB / c mice (purchased from Shanghai Shrek Experimental Animal Company, administered at the age of 6 weeks) were randomly divided into groups after acclimating to the environment. Except for the normal control group, the rest of the animals received imiquimod ointment (5%) External treatment was used to induce the establishment of psoriasis model. The specific method is to shave off the same area of hair on the back of the mice, and apply 62.5 mg of imiquimod ointment daily for seven consecutive days. Animals received different treatment regimens, weighed daily, took orally or externally administered drugs once, and observed disease progression scores. ...
Embodiment 2
[0212] Embodiment 2 (therapeutic effect of Nib1 preparation for external use on animal psoriasis disease model):
[0213] 1. Experimental reagents:
[0214] 5% imiquimod ointment, Aldara, 3M Pharmaceuticals, H20160079; dexamethasone ointment: Shanghai Sanjiu Pharmaceutical. Cetostearyl alcohol was purchased from Hunan Erkang Pharmaceutical, F20170000297.
[0215] 2. Topical preparations:
[0216] There are two formulations designed for external application, and the formulation FLL-15-21-2 is shown in Table 8.
[0217] Table 8: FLL-15-21-2 formulation composition
[0218]
[0219]
[0220] The technical process of the above-mentioned external application formulation FLL-15-21-2:
[0221] 1) Weigh the prescribed amount of API, put it in a beaker, and then add the prescribed amount of acetic acid. When adding, the acetic acid just covers the API powder, and the whole changes from white solid to crystal, which is slightly viscous; then add the prescribed amount of two In ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com