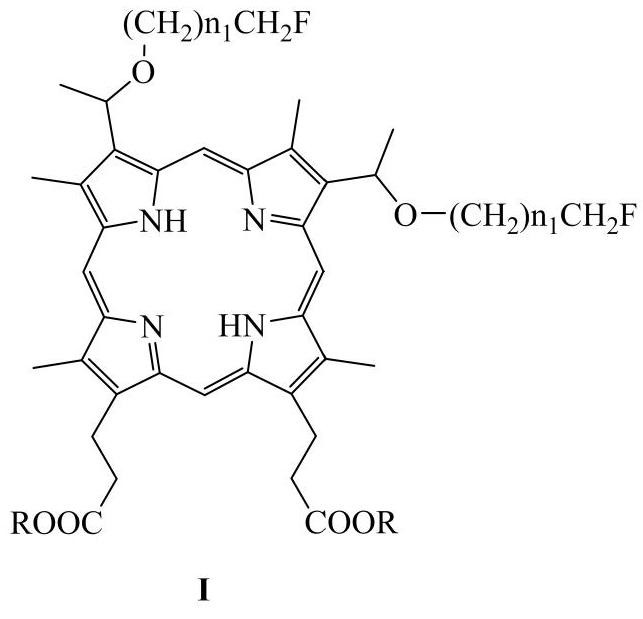
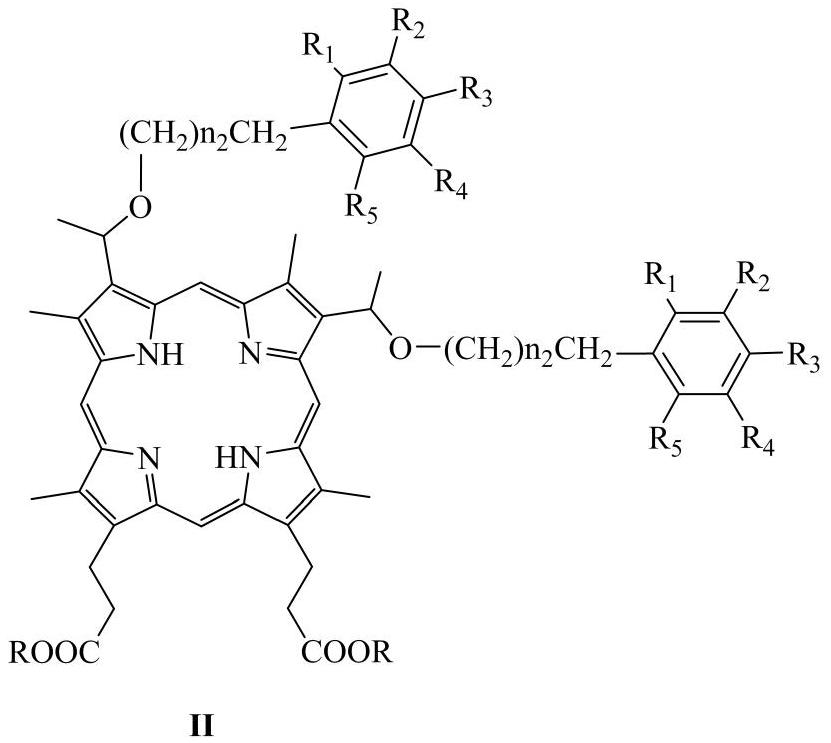
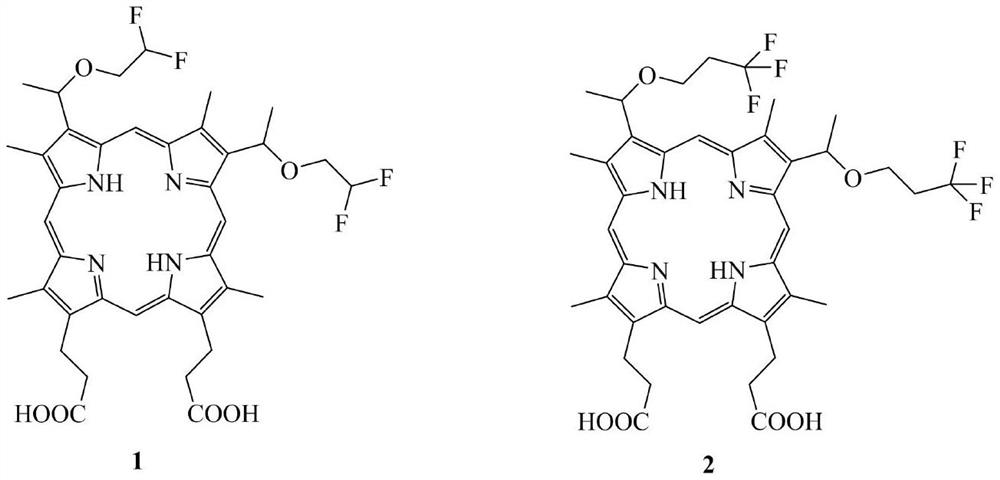
Novel hematoporphyrin monofluoroalkyl diether and fluorophenyl alkyl diether derivatives and application thereof in field of medicines
A technology of hematoporphyrin fluorophenyl alkyl diether and hematoporphyrin monofluoroalkyl diether, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, antitumor drugs, etc., and can solve the problem of unreported Compound pharmacological activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] 3,8-bis(1-(2-fluoroethoxy)ethyl) hypoporphyrin (I 1 ) preparation:
[0094]
[0095]Add protoporphyrin dimethyl ester (614mg, 1.041mmol) into the round bottom flask, then add hydrogen bromide / acetic acid solution (7mL) with a content of about 30%, stir at room temperature for about 36h, after TLC monitors that the reaction is complete, depressurize Remove the solvent by distillation; add 2-fluoroethanol (3 mL) to the residual solid to dissolve it, stir at room temperature for 4 h, add ethyl acetate (EA) to dilute after the reaction, wash with water four times, and remove the solvent by distillation under reduced pressure; (THF, 8mL) to dissolve the residue, add NaOH solution (2M, 3mL) and stir at room temperature for 2h, add water (50mL) to dilute, and add dilute hydrochloric acid solution (2M, 3mL) to adjust the pH to weak acidity, wash with ethyl acetate (50mL ×3) extraction, washed with saturated sodium chloride solution (50mL×3), dried over anhydrous sodium sulf...
Embodiment 2
[0097] 3,8-bis(1-(3-fluoropropoxy)ethyl) hypoporphyrin (I 2 ) preparation:
[0098]
[0099] Compound I 1 The synthetic method prepared compound I 2 . 1 H NMR (400MHz, Pyr-d 5 )δppm: 10.96(s, 1H), 10.89(d, J=10.0Hz, 2H,), 10.39(s, 1H,), 6.29(s, 2H,), 4.69(s, 4H,), 3.94(s ,4H,),3.69(s,12H,),3.65(s,4H,),2.36(s,6H,),1.36-1.19(m,8H,),-3.03(s,2H,). 13 CNMR (101MHz, Pyr-d 5 )δppm:176.92,174.67,151.66,151.01,150.60,137.31,136.91,136.67,136.28,125.28,124.93,124.69,124.25,100.65,100.40,100.10,99.33,99.03,83.90,82.28,75.19,66.64,39.23,33.41 ,33.09,32.90,31.27,30.89,26.86,24.23,23.82,22.69,15.57,14.12,14.07,12.94,12.91,12.81,12.76. 19 F NMR (377MHz, DMSO-d 6 )δppm: 17.66(s).MS(MALDI TOF)(DART Positive):m / z calcd for C 40 h 48 f 2 N 4 o 6 [M+H] + , 719.6; found, 719.6.
Embodiment 3
[0101] 3,8-bis(1-(4-fluorobutoxy)ethyl) hypoporphyrin (I 3 ) preparation:
[0102]
[0103] Compound I 1 The synthetic method prepared compound I 3 . 1 H NMR (400MHz, Pyr-d 5 )δppm: 11.00(s,1H,),10.94(s,1H,),10.89(s,1H,),10.40(s,1H,),6.27(s,2H,),4.69(s,4H,) ,4.51(s,2H,),4.39(s,2H,),3.83(s,2H,),3.78–3.69(m,12H,),3.67(s,2H,),3.64(s,4H,) ,2.37(s,6H,),1.95(s,4H,),-3.02(s,2H,). 13 CNMR (101MHz, Pyr-d 5 )δppm:175.39,150.17,149.08,139.72,135.78,135.38,135.14,134.76,123.76,123.64,123.41,123.17,122.73,99.04,98.71,97.49,96.25,84.75,83.12,73.50,68.79,37.70,27.87,27.68 ,26.22,25.41,22.30,11.45,11.40,11.34,11.26. 19 F NMR (377MHz, Pyr-d 5 )δppm: -216.99(d,J=24.5Hz),-217.08(s),-217.12--217.34(m).MS(MALDI TOF)(DART Positive):m / z calcdfor C 42 h 52 f 2 N 4 o 6 [M+H] + ,746.8; found, 746.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com