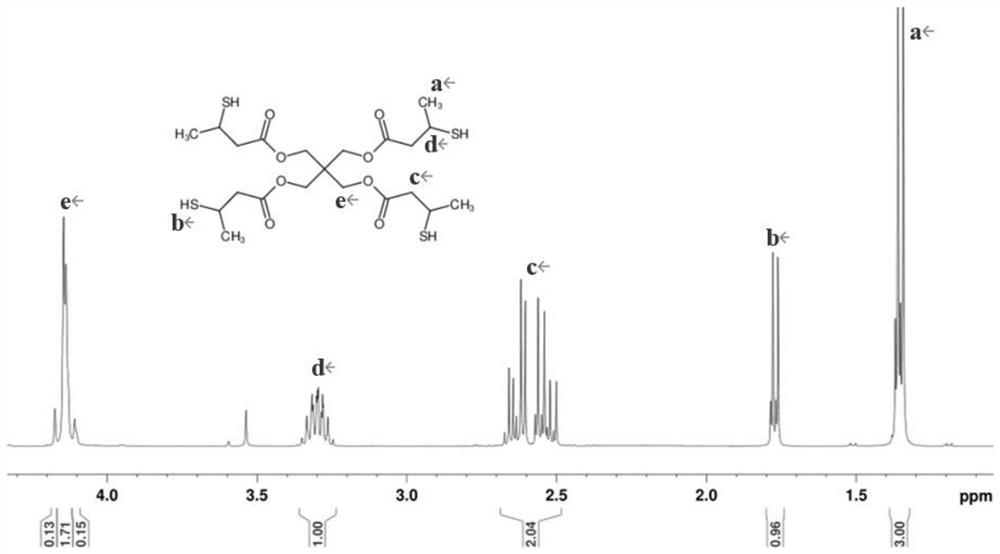
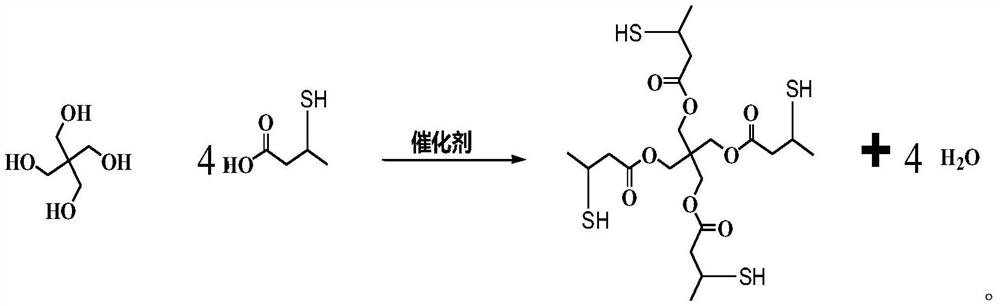
Preparation method of pentaerythritol ester
A technology for pentaerythritol ester and pentaerythritol is applied in the field of preparation of pentaerythritol ester, and can solve the problems of many by-products, difficult recycling of catalysts, and difficulty in achieving industrial application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Catalyst H 3 PW 12 o 40 -Methanesulfonic acid / Mesoporous TiO 2 Preparation of:
[0057] A) 30.5 g of template agent P12 and 5 g of isopropyl titanate were stirred in ethanol for 1 h and mixed evenly to obtain the first mixed solution;
[0058] B) the first mixed solution and heteropolyacid H 3 PW 12 o 40 1 g, 1 g of methanesulfonic acid were stirred for 1 h and mixed uniformly to obtain the second mixed solution;
[0059] C) volatilize the second mixed solution at 40° C. for 12 hours to remove the solvent to obtain a gel;
[0060]D) Aging at 100°C for 12h, and then calcining at 350°C for 2h to remove the template agent and obtain catalyst H 3 PW 12 o 40 -Methanesulfonic acid / Mesoporous TiO 2 . The particle size of the catalyst is 3nm and the specific surface area is 200m 2 / g.
[0061] 2, the preparation of pentaerythritol ester
[0062] (1) In the four-neck flask equipped with mechanical stirring, thermometer, reflux condenser and water trap, add 41.2...
Embodiment 2
[0072] 1. Catalyst H 3 SiW 12 o 40 -Sulphamic acid / mesoporous TiO 2 Preparation of:
[0073] A) 31 g of template agent P12 and 8 g of n-butyl titanate were stirred in methanol for 0.5 h and mixed uniformly to obtain the first mixed solution;
[0074] B) the first mixed solution and heteropolyacid H 3 SiW 12 o 40 3g, 1g of sulfamic acid were stirred for 1h and mixed uniformly to obtain the second mixed solution;
[0075] C) volatilize the second mixed solution at 40° C. for 13 hours to remove the solvent to obtain a gel;
[0076] D) Aging at 100°C for 14h, and then calcining at 400°C for 2h to remove the template agent to obtain catalyst H 3 SiW 12 o 40 -Sulphamic acid / mesoporous TiO 2 . The particle size of the catalyst is 3.5nm and the specific surface area is 180m 2 / g.
[0077] 2, the preparation of pentaerythritol ester
[0078] (1) In the four-necked flask that mechanical stirring, thermometer, reflux condenser and water separator are housed, add 41.27g of ...
Embodiment 3
[0086] 1. Catalyst H 3 PW 12 o 40 -Methanesulfonic acid / mesoporous SiO 2 Preparation of:
[0087] A) 30.8 g of template agent P12 and 7 g of ethyl orthosilicate were stirred in acetonitrile for 1.5 h and mixed uniformly to obtain the first mixed solution;
[0088] B) the first mixed solution and heteropolyacid H 3 PW 12 o 40 2g and 2g of methanesulfonic acid were stirred for 1h and mixed uniformly to obtain the second mixed solution;
[0089] C) volatilize the second mixed solution at 40° C. for 10 h to remove the solvent to obtain a gel;
[0090] D) aging at 100°C for 12h, and then calcining at 400°C for 1.5h to remove the template agent and obtain catalyst H 3 PW 12 o 40 -Methanesulfonic acid / mesoporous SiO 2 . The particle size of the catalyst is 4nm and the specific surface area is 210m 2 / g.
[0091] 2, the preparation of pentaerythritol ester
[0092] (1) In the four-neck flask equipped with mechanical stirring, thermometer, reflux condenser and water sepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com