Platform bacterium for producing L-aspartate, recombinant bacterium constructed based on platform bacterium for producing beta-alanine and construction method of recombinant bacterium
A technology of aspartate aminotransferase and aspartic acid, which is applied in the field of microorganisms, can solve problems such as unsuitable separation and purification, harsh reaction conditions of chemical synthesis methods, and environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0231] Embodiment 1, the construction of genetic engineering strain
[0232] 1. Construction of a recombinant plasmid expressing Escherichia coli aspartate aminotransferase (i.e. recombinant plasmid pLB1a-E)
[0233] 1. The bacterial genome extraction kit was used to extract the genomic DNA of Escherichia coli BW25113.
[0234] 2. Using the Escherichia coli genomic DNA extracted in step 1 as a template, using aspC-GF and aspC-GR as primers, perform PCR amplification with high-fidelity TransStart FastPfu DNA polymerase to obtain PCR amplification products.
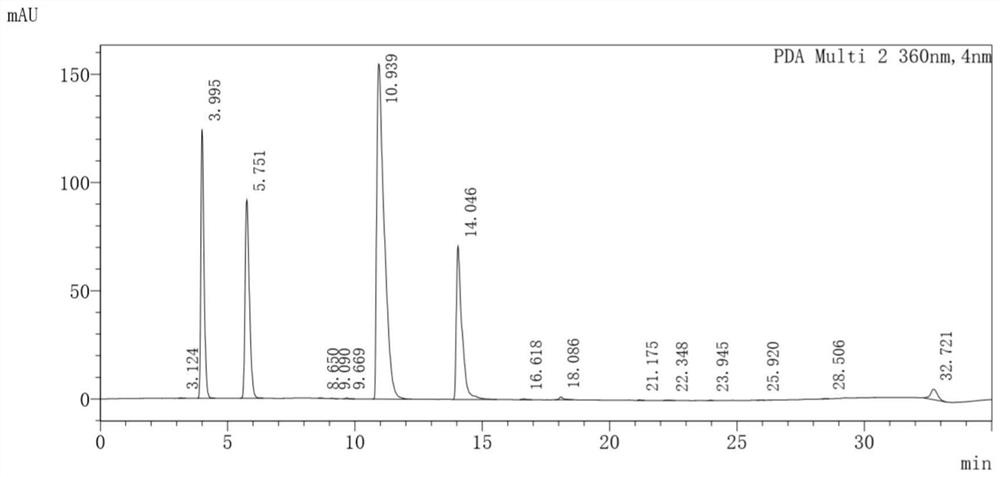
[0235] 3. Perform agarose gel electrophoresis on the PCR amplification product obtained in step 2, and then recover a DNA fragment of about 1240 bp. The DNA fragment contains the aspC gene, and the nucleotide sequence of the aspC gene is shown as sequence 2 in the sequence list. The aspC gene encodes the aspartate aminotransferase of E. coli.
[0236] 4. Digest the vector pLB1a with restriction endonucleases NcoI and Xho...
Embodiment 2
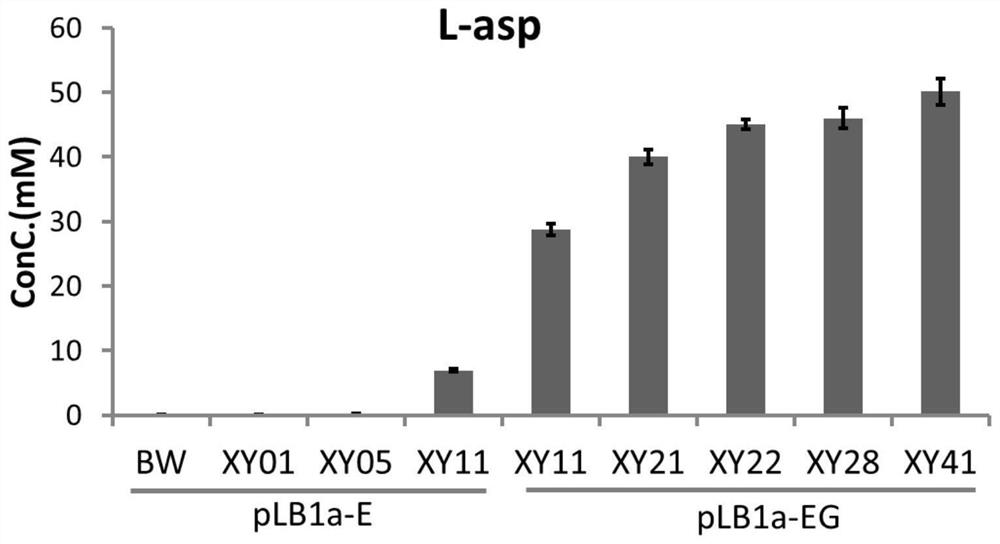
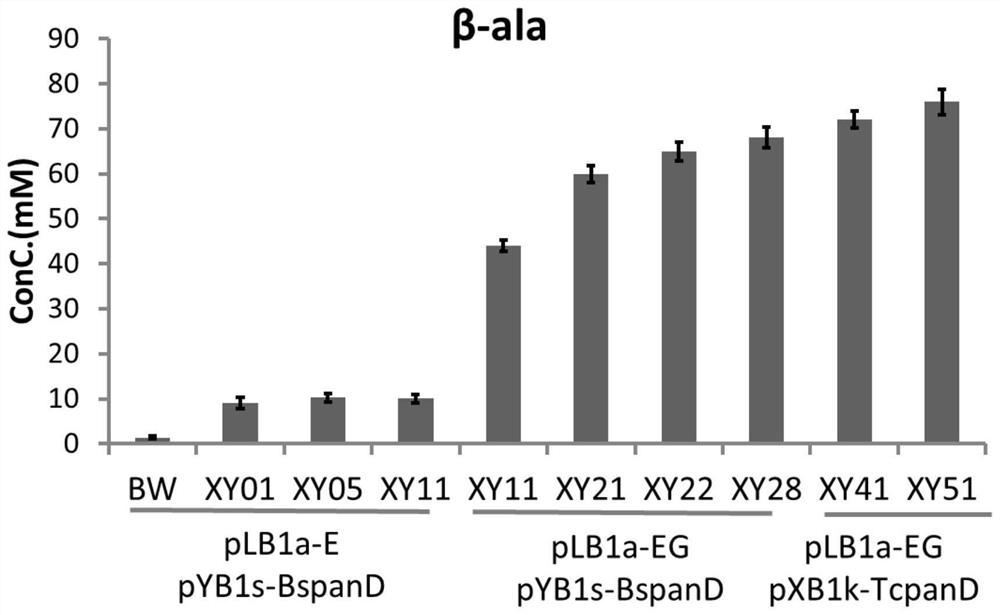
[0425] Embodiment 2, utilize the genetic engineering bacterial strain that embodiment 1 constructs to prepare L-aspartic acid or β-alanine
[0426] Autoinduction medium ZYM consisted of 100 mL of solution A, 2 mL of solution B, 2 mL of solution C, 200 μL of solution D, and 100 μL of solution E.
[0427] Solution A: an aqueous solution containing 1% (m / m) tryptone and 0.5% (m / m) yeast powder.
[0428] Solution B: Contains 1.25M Na 2 HPO 4 、1.25M KH 2 PO 4 , 2.5M NH 4 Cl and 0.25M Na 2 SO 4of aqueous solution.
[0429] Solution C: an aqueous solution containing 25% (m / m) glycerol, 2.5% (m / m) glucose and 10% (m / m) L-arabinose.
[0430] Solution D: MgSO at a concentration of 1M 4 aqueous solution.
[0431] Solution E: Contains 50mM FeCl 3 , 20mM CaCl 2 , 10mM MnCl 2 , 10mM ZnSO 4 , 2mM CoCl 2 , 2mMNiCl 2 , 2mM Na 2 Mo 4 , 2mM Na 2 SeO 3 and 2mM H 3 BO 3 of aqueous solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com