Method for detecting fluorobromomethane residues in bulk drugs
A kind of fluorobromomethane and detection method technology, applied in the field of analysis, can solve the problems of fluorobromomethane residue detection not being reported and included and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
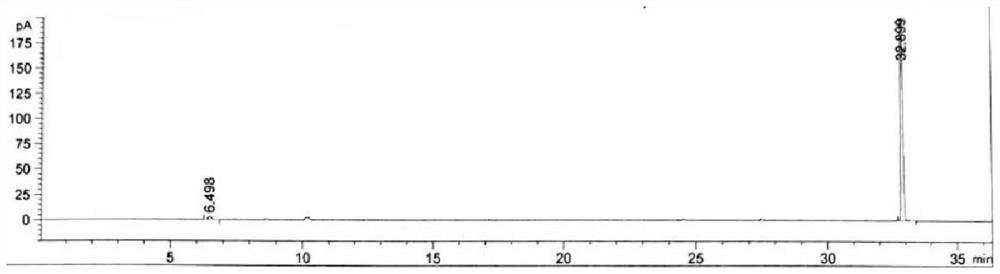
[0053] Embodiment 1 specificity test
Embodiment 11
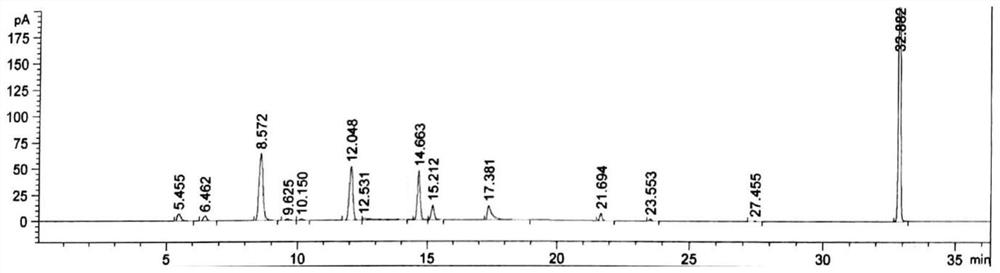
[0055] Take methanol, acetone, acetonitrile, tetrahydrofuran, n-hexane, toluene, bromofluoromethane, dichloromethane, diethylamine, triethylamine, ethyl acetate, dimethylacetamide (DMA), and mesityl oxide respectively. Mix the API limit of each solvent by 100 times, add to N-methylpyrrolidone solvent and mix, as a specific mixed sample solution, take 2ml and put it in a 20ml headspace bottle, seal it for later use;
[0056] The above solutions were injected separately, and the chromatographic conditions were as follows:
[0057] Using gas chromatography, using DB-624 as a chromatographic column (6% cyanopropylphenyl-94% dimethyl polyoxysilane, 30m × 0.53mm × 3.0μm), the carrier gas is helium, and the carrier gas flow rate is 2.0 ml / min; use the headspace sampling method to inject samples, the headspace temperature is 30°C; the headspace time is 30min; the injection temperature is 200°C; the column temperature programming method is adopted, and the column temperature increase pro...
Embodiment 12
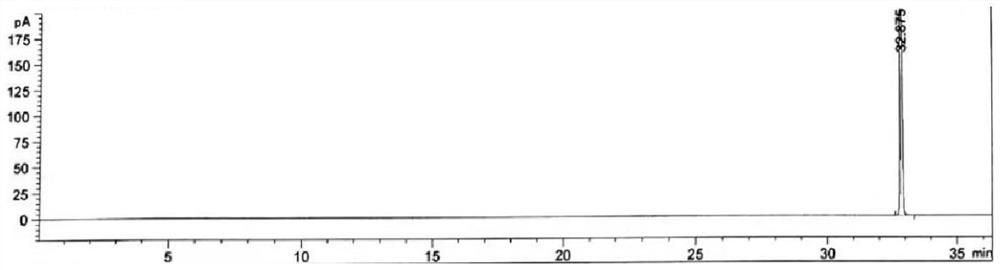
[0064] Take methanol, acetone, acetonitrile, tetrahydrofuran, n-hexane, toluene, bromofluoromethane, dichloromethane, diethylamine, triethylamine, ethyl acetate, dimethylacetamide (DMA), and mesityl oxide respectively. Mix the API limit of each solvent by 100 times, add to N-methylpyrrolidone solvent and mix, as a specific mixed sample solution, take 2ml and put it in a 20ml headspace bottle, seal it for later use;
[0065] The above solutions were injected separately, and the chromatographic conditions were as follows:
[0066] Using gas chromatography, using DB-624 as a chromatographic column (6% cyanopropylphenyl-94% dimethylpolyoxysilane, 30m × 0.53mm × 3.0μm), the carrier gas is helium, and the carrier gas flow rate is 2.2 ml / min; use the headspace sampling method to inject samples, the headspace temperature is 35°C; the headspace time is 28min; The column temperature was 35°C, maintained for 12.0 minutes, raised to 200°C at a rate of 8°C per minute, and maintained for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com