Dual drug composition, capsule drug, tablet drug and application of dual drug composition
A composition and drug technology, applied in the directions of drug combination, capsule delivery, pill delivery, etc., can solve the problems of increased risk of thrombus, difficult to control, and high allergic reaction rate, and achieve the effect of alleviating nervous system toxicity and small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] The preparation of embodiment one capsule medicine
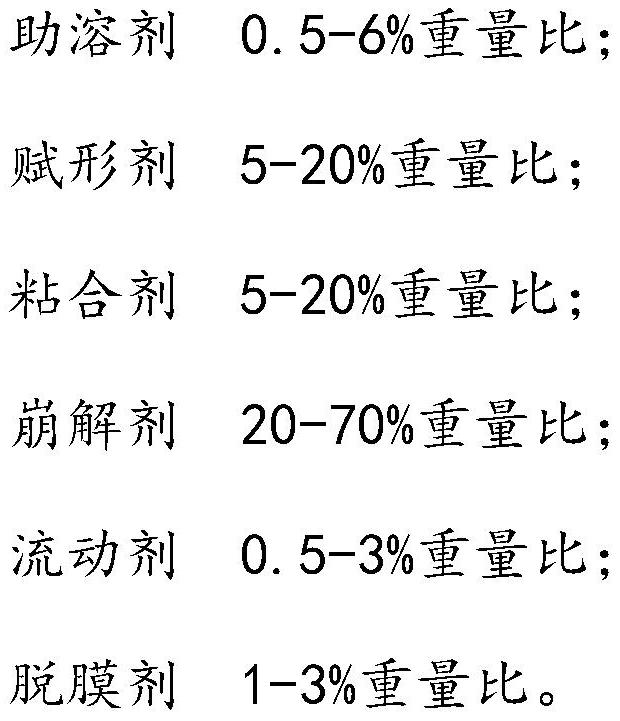
[0142] Net weight of capsule content: 0.20g / capsule, that is, 200mg / capsule
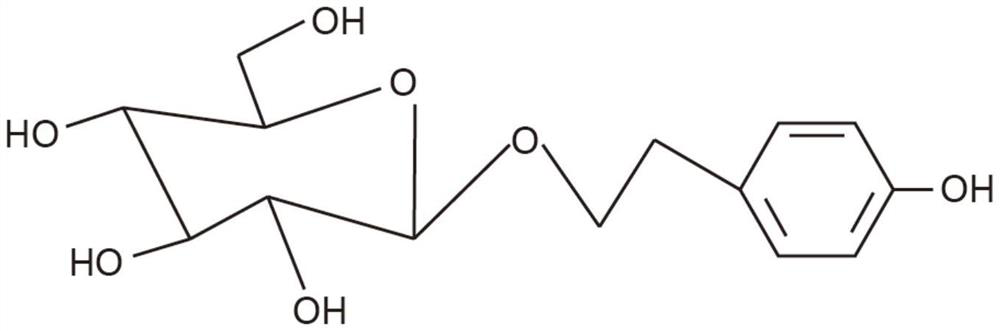
[0143] Saliclin extract: 50mg Saliclin content: 3%
[0144] Shikonin B extract: 150mg Shikonin B content: 7%
[0145] Each capsule contains salicillin: 50mg*3%=1.5mg
[0146] Each capsule contains shikonic acid B: 150mg*7%=10.5mg
[0147]
Embodiment 2
[0149] Net weight of capsule contents: 0.40g / capsule, that is, 400mg / capsule
[0150] Saliclin extract: 100mg Saliclin content: 3%
[0151] Shikonin B extract: 300mg Shikonin B content: 7%
[0152] Each capsule contains salicillin: 100mg*3%=3mg
[0153] Each capsule contains shikonic acid B: 300mg*7%=21mg
[0154]
Embodiment 3
[0156] Net weight of capsule contents: 0.60g / capsule, that is, 600mg / capsule
[0157] Saliclin extract: 150mg Saliclin content: 3%
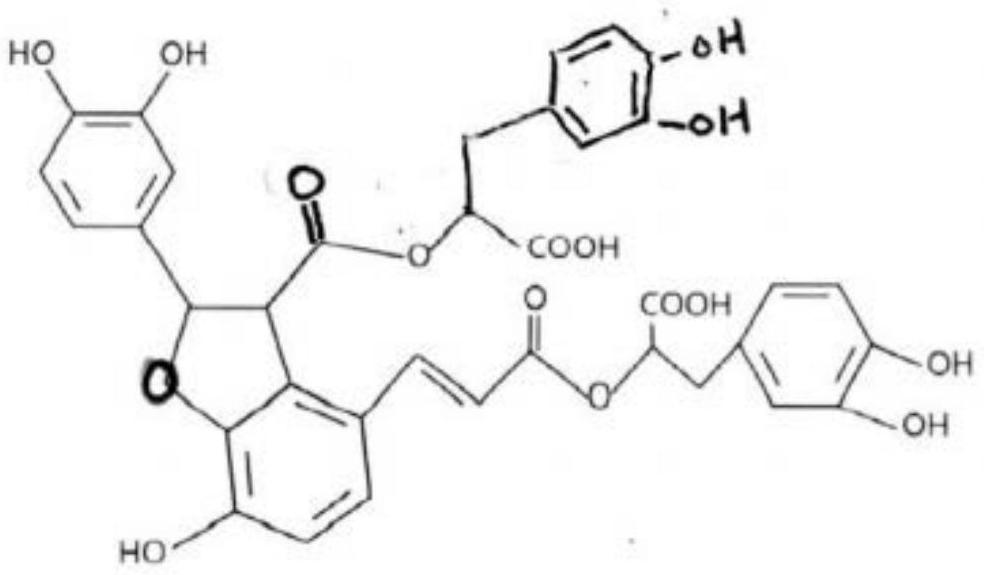
[0158] Shikonin B extract: 450mg Shikonian acid content: 6%
[0159] Each capsule contains salicillin: 150mg*3%=4.5mg
[0160] Each capsule contains shikonian acid B: 450mg*6%=27.0mg
[0161]
[0162]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com