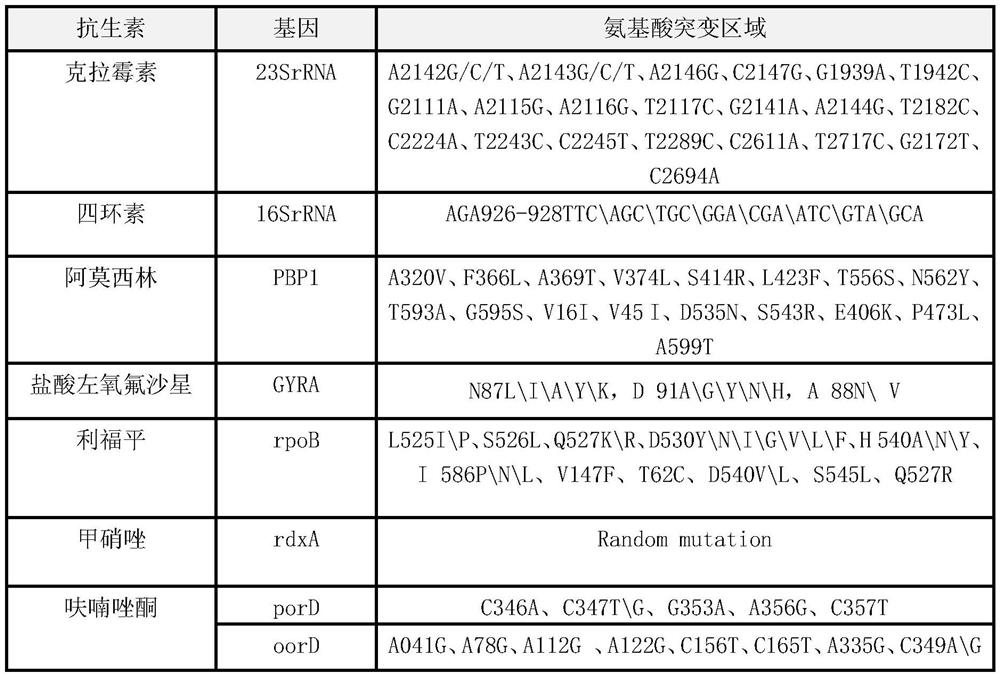
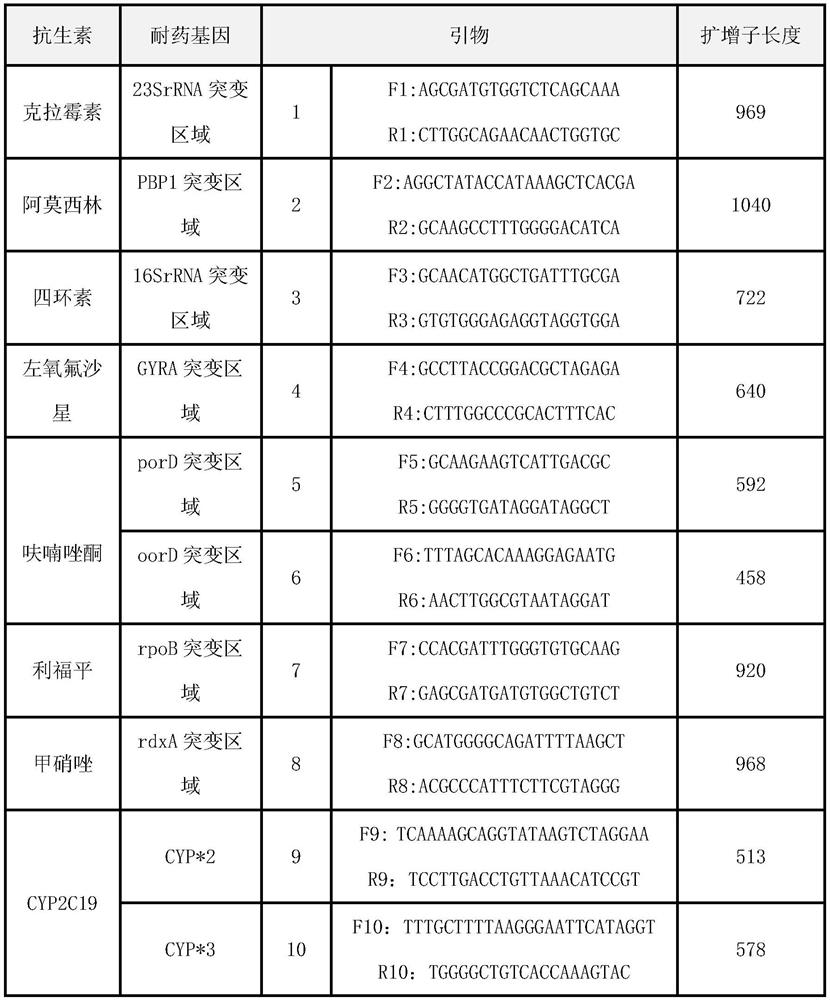
Quick molecular diagnosis method and reagent kit for individualized medication guidance for eradication therapy of helicobacter pylori
A technology of Helicobacter pylori and a diagnostic method, applied in the field of diagnostic methods and kits for detecting and judging drug resistance of Helicobacter pylori, can solve the problems of low culture success rate, poor patient compliance, long culture cycle, etc., and achieves the operation process. Simple, reasonable design, fast detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Helicobacter pylori genome extraction process in gastric biopsy samples
[0065] 1. Sampling requirements:
[0066] 1) Confirm that the patient stopped taking antibiotics, proton pump inhibitors, and bismuth for more than four weeks before the test, and Chinese medicine for more than two weeks, and was verified positive by rapid urease test paper and urea breath test (UBT).
[0067] 2) When taking gastroscopy biopsy samples, try to take double samples of gastric antrum and gastric body lesions, put them into the transport medium test tube, and transport them under refrigeration at 2-8 degrees within 12 hours.
[0068] 3) The HP of newly treated patients is mostly distributed in the gastric antrum 2-5cm away from the pylorus, and the HP of patients undergoing salvage treatment will transfer from the gastric antrum to the gastric body and gastric fundus, so samples from the gastric fundus and gastric body should be collected at the same time for the latter .
...
Embodiment 2
[0086] Embodiment 2PCR amplification:
[0087] ddH 2 O is used as negative control, and Helicobacter pylori standard strain 26695 and 43504 are used as positive control; PCR amplification reagent can use reagent TaKaRaTaq TM Version 2.0 (TaKaRa, Code No.R004A) (Takara), is a ready-to-use PCR premix solution, which can be amplified by simply adding primers and templates to simplify the experimental steps.
[0088] 1.PCR primer amplification:
[0089] Using TaKaRaTaq TM Version 2.0 (TaKaRa, Code No.R004A), cold start method, amplified according to the components shown in Table 5 and the procedures shown in Table 6. The products were numbered P1-10, and stored at low temperature.
[0090] table 5
[0091] Reagent Usage amount DNA template (<500ng / μl)
1μl F Primer (10μM) 1μl R Primer (10μM) 1μl Premix Taq TM
25μl Nuclease-Free Water 22μl Total 50μL
[0092] Table 6
[0093]
[0094] 2. Nested primer amplifica...
Embodiment 3
[0102] Embodiment 3 sequencing part:
[0103] 1. PCR amplification
[0104] Using corresponding primers, use Tianjin Qingke 2×TsingKE Master Mix (Code No.: TSE003) system for PCR amplification, and ddH 2 O was used as a negative control, amplification was performed with the reaction components shown in Table 10, and 2 μl was taken for 1% agarose gel electrophoresis.
[0105] Table 10
[0106]
[0107] 2.PCR product purification
[0108] 1) Balance and centrifuge the checked PCR samples (4000rpm), check the volume of each sample, and replenish water to 50 μl.
[0109] 2) According to the sample: 6×Loading buffer=5:1, add 10 μl of 6×Loading buffer, if the sample volume is 50-70 μl, add 5 μl of 6×Loading buffer, and centrifuge and mix at 4000 rpm.
[0110]3) Put the sample into the pre-prepared 1.2% purified gel. The sample pointing order is: 1-8 wells in the first row of the purified gel are placed into the first column of the sample, the 9th hole is a marker, and the 10-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com