Preparation method and application of impurities of 4-aminoquinoline compounds
A technology of hydroxyquinoline and mixture, applied in the field of organic chemical synthesis, can solve the problem of difficulty in obtaining high-purity LKL-4 isomers and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
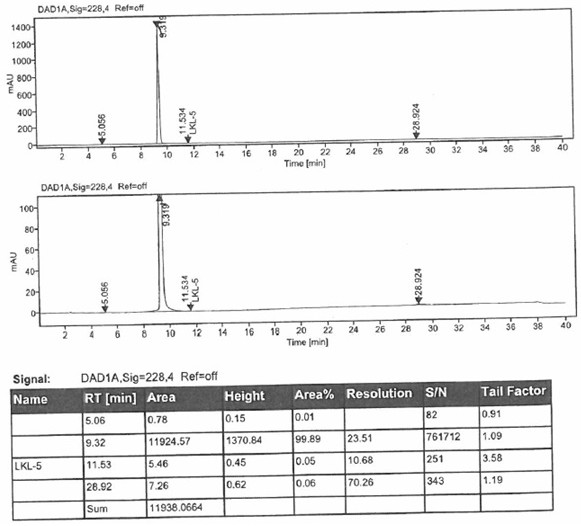
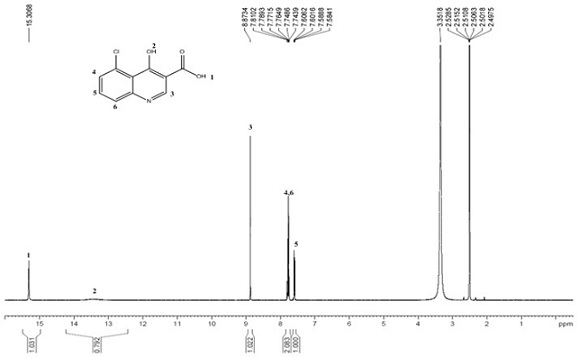
[0040] Preparation of 5-chloro-4-hydroxy-3-quinolinecarboxylic acid (LKL-5 isomer)
[0041] Dissolve 3 g of mixture 1 (the purity of 5-chloro-4-hydroxy-3-quinoline carboxylic acid is 8.5%) in dimethyl sulfoxide to prepare a solution with a concentration of 150 mg / ml, and separate by preparative liquid chromatography , the chromatographic conditions are as follows:
[0042] The chromatographic column is a C18 column with isocratic elution, and the mobile phase is acetonitrile-phosphate aqueous solution (pH=4.5), where the volume ratio of acetonitrile and phosphate aqueous solution is 1:4, the flow rate is 30ml / min, and the column temperature is 30°C , the injection volume is 3ml. 5-Chloro-4-hydroxy-3-quinolinecarboxylic acid was collected by elution with a purity of 99.21%.
Embodiment 2
[0044] Preparation of 5-chloro-4-hydroxy-3-quinolinecarboxylic acid (LKL-5 isomer)
[0045] Mix and stir 3g (0.01mol) of LKL-3 and 10ml of paraffin oil, add 2.94g (0.03mol) of concentrated sulfuric acid once at 20~30°C, continue to stir and heat to 100°C for 2h. Cool to 20~30°C, filter under reduced pressure to obtain a brownish-yellow solid, wash the filter cake with 10ml water, 10ml chloroform and 10ml ethanol in sequence, and continue to filter for 10min, the obtained off-white solid mixture 2 (LKL-4 isomer purity 70.4%). The above-mentioned LKL-4 isomer was directly mixed with 10ml of 25% aqueous sodium hydroxide solution, stirred at 20~30°C for 2h, the reaction solution was filtered under reduced pressure and washed with 5ml of ethanol, dried to obtain off-white solid mixture 1 (LKL -5 isomer purity 75.3%). The above mixture 1 was dissolved in dimethyl sulfoxide to form a solution with a concentration of 120 mg / ml, and separated by liquid phase preparative chromatograph...
Embodiment 3
[0050] Preparation of 5-chloro-4-hydroxy-3-quinolinecarboxylic acid (LKL-5 isomer)
[0051] Mix and stir 3g (0.01mol) of LKL-3 and 18ml of paraffin oil, add 4.45g (0.045mol) of concentrated sulfuric acid once at 20~30°C, continue to stir and heat to 90°C for 2.5h. Cool to 20~30°C, filter under reduced pressure to obtain a brownish-yellow solid, wash the filter cake with 10ml water, 10ml chloroform and 10ml ethanol in sequence, and continue to filter for 10min, the obtained off-white solid mixture 2 (LKL-4 isomer purity 72.2%). The above-mentioned LKL-4 isomer was directly mixed with 10ml of 30% potassium hydroxide aqueous solution, stirred at 20~30°C for 2h, the reaction solution was filtered under reduced pressure and washed with 5ml of ethanol, dried to obtain off-white solid mixture 1 (LKL -5 isomer purity 76.1%). The above mixture 1 was dissolved in dimethyl sulfoxide to prepare a solution with a concentration of 150 mg / ml, and separated by preparative liquid chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com