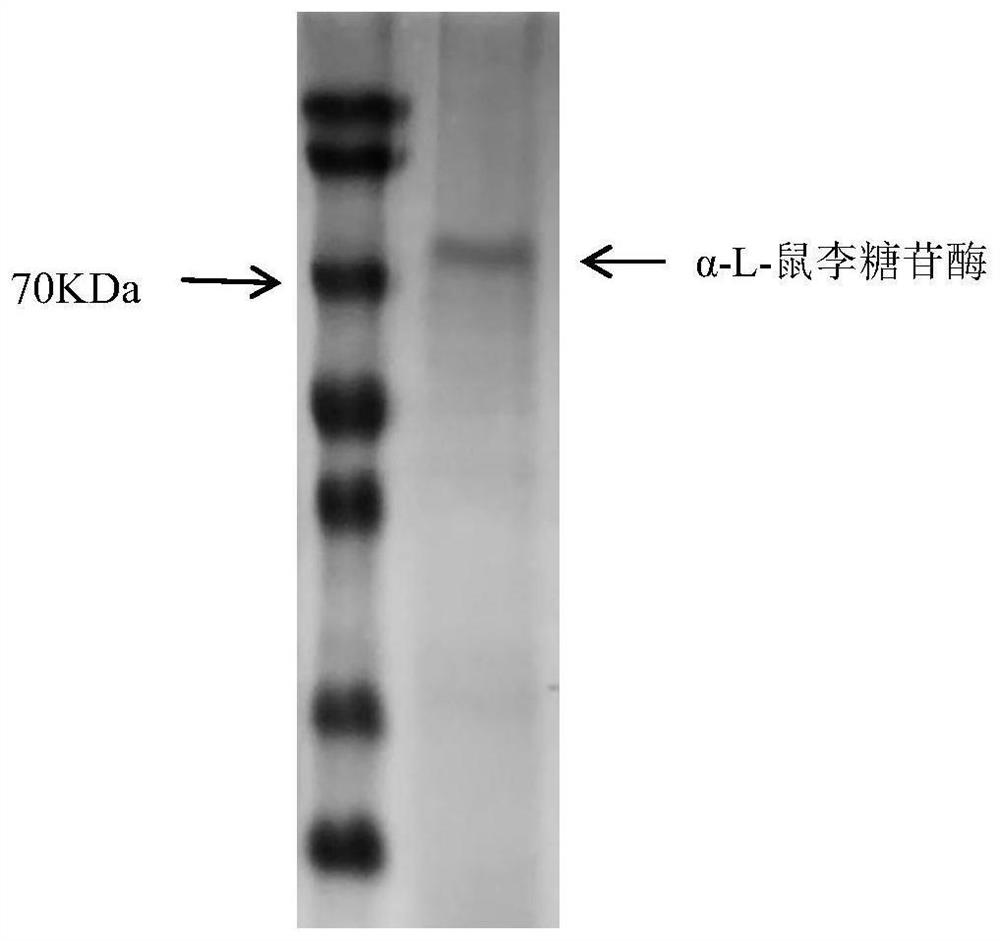
Aspergillus niger Rha-N1 and application thereof
A technology of rha-n1 and Aspergillus niger, which is applied in the fields of genetic engineering and biomedicine, can solve the problems of difficulty in achieving purity, difficulty in separating other enzyme components, low expression level, etc., and achieves environmental protection, mild reaction conditions, and operation. simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
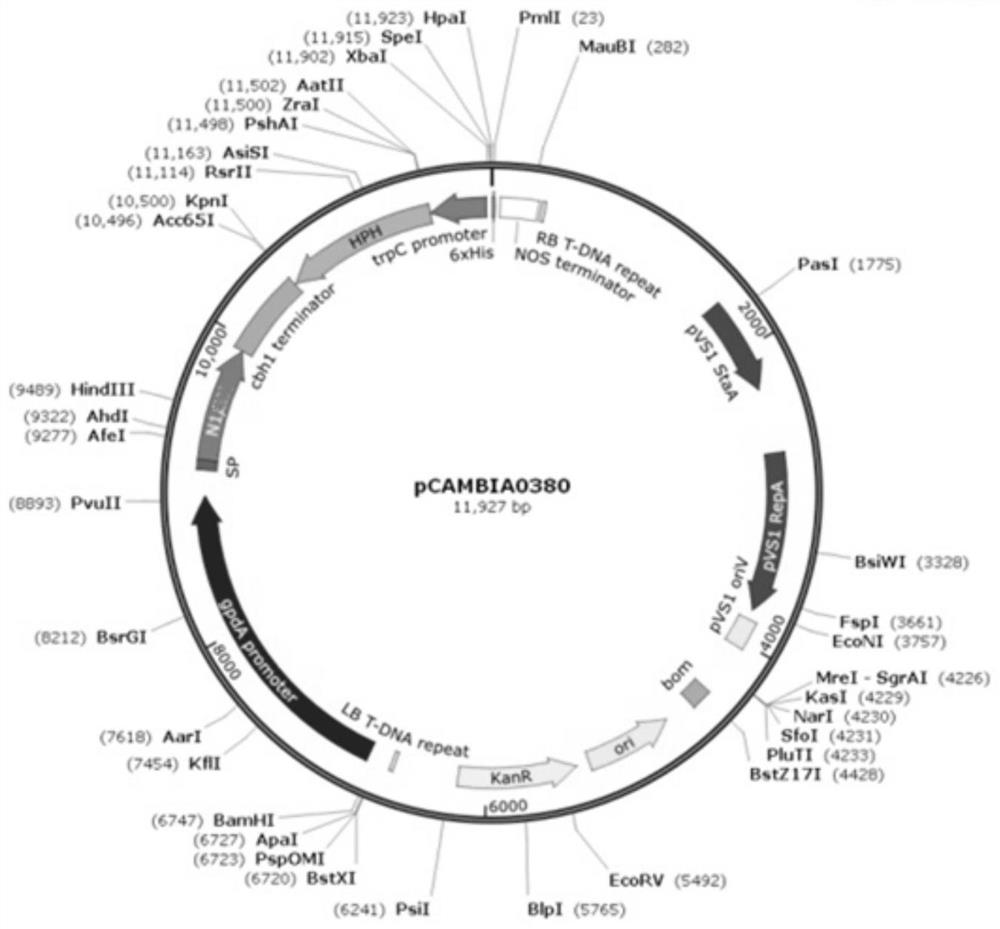
[0044] Example 1 α-L-rhamnosidase gene synthesis and recombinant plasmid construction
[0045] The α-L-rhamnosidase gene (GenBank: XP_001389086.1) from Aspergillus niger searched by NCBI was synthesized by Qingke Company. The final gene sequence is shown in SEQ ID NO.2, encoding The amino acid sequence is shown in SEQ ID NO.1.
[0046] Primer pairs designed to amplify the α-L-rhamnosidase gene sequence with homology arms,
[0047] N1-S: CCGCTTGAGCAGACATCACAATGTGGTCTTCCTGGCTGCTGTC
[0048] N1-N: GGCTTTCGCCACGGAGCTCTAGTGGTGGTGGTGGTGGTGATTAT
[0049] Using the synthetic sequence of SEQ ID NO.2 as a template, PCR amplification was performed with the primer pair (N1-S and N1-N), and Primer Star MasterMix (Takara Company) high-fidelity enzyme was selected for pre-denaturation at 98°C for 3 minutes ; Amplification stage 30 cycles, according to 98 ℃, 10s, 55 ℃, 5s, 72 ℃, 15s; extension 72 ℃, 10min. The PCR product was digested and purified to obtain a purified fragment (the target...
Embodiment 2
[0055] Embodiment 2 Agrobacterium Transformation
[0056] Take out Agrobacterium tumefaciens competent cells AGL-1 from the -80°C refrigerator, thaw in an ice bath, add 10 μl of the recombinant plasmid prepared in Example 1, bathe in ice for 5 minutes, freeze in liquid nitrogen for 5 minutes, heat shock at 37°C for 5 minutes, and store on ice. After bathing for 5 minutes, add 1mL LB liquid medium, incubate at 28°C for 2h, spread on LB solid plates containing 50μg / mL kanamycin and 50μg / mL rifampicin, and incubate at 28°C for 2d.
[0057] Pick out a single colony that grows well on the plate, and suspend it in 10 μL ddH 2 O made bacterial suspension, boiled water bath 10min, cooled to room temperature, used it as a template, identified positive clones by colony PCR method, and designed primer pairs for verifying the target gene,
[0058] YZ-S: CCCGCTTGAGCAGACATCAC
[0059] YZ-N: CCGTCATGATACGGGCTCAC
[0060] The bacterial suspension was used as a template, and the primer pair...
Embodiment 3
[0061] Embodiment 3 Agrobacterium-mediated transformation of Aspergillus niger
[0062] (1) Aspergillus niger host bacteria (purchased from Yiyuan Kangyuan Biotechnology Co., Ltd., product number 3.350) were inoculated into PDA slant medium, and cultured at 30°C for 5 days. Wash the spores from the slope with 10 mL of sterile water and filter with two layers of sterile lens tissue to obtain a spore suspension. Measure the spore concentration with a cytometer and dilute it to 10 7 individuals / mL to obtain a suspension of Aspergillus niger spores for Agrobacterium transformation.
[0063] (2) Pick a single colony of Agrobacterium that has been transferred to the recombinant plasmid from the LB plate of Example 2, and inoculate it into the LB liquid medium containing 50 μg / mL kanamycin and 50 μg / mL rifampin, at 28° C. , shake culture at 200rpm for 24h; centrifuge the culture solution, and dilute the bacteria to OD with AIM liquid medium 600 About 0.4, 28°C, 200rpm continue to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com