Synthesis process of 4-amino-6-hydroxybenzyl bromide
The technology of benzyl hydroxybromide and synthesis process is applied in the field of synthesis technology of photoinitiator, and can solve the problems of toxicity of photoinitiator and its photolysis products, low jumping efficiency between initiator systems, low molar extinction coefficient, etc., Achieve the effect of highlighting the photo-initiated activity, low cost, and simplifying the process operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
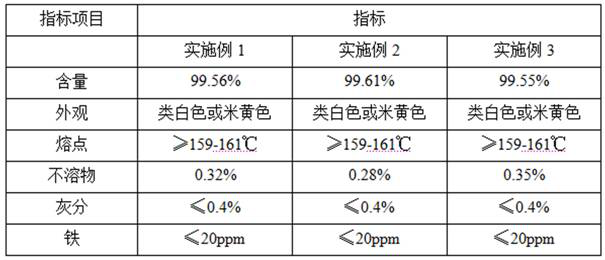
Embodiment 1
[0021] A kind of 4-amino-6-hydroxyl benzyl bromide synthetic technique, comprises the following steps:
[0022] A. Hydrogenation reaction: put 400 parts of 5-nitro-o-cresol, 0.5 parts of PdC catalyst and 2000 parts of methanol into the autoclave, replace it with hydrogen three times, fill it with 1.0MPa hydrogen and set the temperature to 66 °C, Start to stir and heat up, and circulate the cooling water through the stirring sleeve. During the hydrogenation reaction, when the detection pressure drops to 0.5-0.6MPa, it is time to replenish the hydrogen pressure to 1.0MPa. After repeating this operation 4 times, when the pressure drops to 0.8MPa , add hydrogen once again, continue to react for 3 hours after repeating twice, the reduction reaction is basically completed, and the crude product of 5-amino-o-cresol is obtained, and the crude product of 5-amino-o-cresol is sucked out by a vacuum pump, and the crude product of 5-amino-o-cresol is Carry out cooling and sampling, and det...
Embodiment 2
[0027] A kind of 4-amino-6-hydroxyl benzyl bromide synthetic technique, comprises the following steps:
[0028] A. Hydrogenation reaction: put 400 parts of 5-nitro-o-cresol, 0.8 parts of PdC catalyst and 2500 parts of methanol into the autoclave, replace it with hydrogen three times, fill it with 1.0 MPa hydrogen and set the temperature to 66 °C, Start to stir and heat up, and circulate the cooling water through the stirring shaft sleeve. During the hydrogenation reaction, when the detection pressure drops to 0.5-0.6MPa, it is time to replenish the hydrogen pressure to 1.0MPa. After repeating this operation 5 times, when the pressure drops to 0.8MPa , add hydrogen once again, continue to react for 4 hours after repeating twice, and the reduction reaction is basically completed to obtain the crude product of 5-amino-o-cresol. Carry out cooling and sampling, and detect that the conversion rate of raw materials is greater than 99.5%;
[0029] B. Pressure filtration and decoloriz...
Embodiment 3
[0033] A kind of 4-amino-6-hydroxyl benzyl bromide synthetic technique, comprises the following steps:
[0034] A. Hydrogenation reaction: Put 400 parts of 5-nitro-o-cresol, 1.0 parts of PdC catalyst and 3000 parts of methanol into the autoclave, replace it with hydrogen three times, fill it with 1.0 MPa hydrogen and set the temperature to 66 ° C, Start to stir and heat up, and circulate the cooling water through the stirring shaft sleeve. During the hydrogenation reaction, when the detection pressure drops to 0.5-0.6MPa, it is time to replenish the hydrogen pressure to 1.0MPa. After repeating this operation 5 times, when the pressure drops to 0.8MPa , add hydrogen once again, continue to react for 5 hours after repeating twice, the reduction reaction is basically completed, and the crude product of 5-amino-o-cresol is obtained, and the crude product of 5-amino-o-cresol is sucked out by a vacuum pump, and the crude product of 5-amino-o-cresol is Carry out cooling and sampling,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com