Medicine composition of R848 or medicine composition of combination of R848 and sorafenib for treating cancer and application
A combination therapy, R848 technology, applied in the field of pharmaceutical compositions for the treatment of cancer, can solve the problems of easy drug resistance, low therapeutic efficacy, and many side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1. Establishment of mouse tumor model
[0058] Hep1-6 cells (liver cancer cell line preserved in the applicant's cell room) were stored in DMEM supplemented with 10% fetal bovine serum at 37°C and 5% CO 2 cultivated under conditions. Hep1-6 cells were subcutaneously inoculated into C57 mice (purchased from Beijing Weitong Lihua Company, male mice, 4-5 weeks old, weighing about 20 g), and each mouse was inoculated with 1×10 6 cells (day 0). Cells were allowed to grow into tumors, and mice were then randomly divided into four groups (n=10): 1) control group, 2) sorafenib group, 3) R848 group, and 4) sorafenib+R848 combination Each group was given the corresponding drug or vehicle (from the 10th day to the 31st day).
Embodiment 2
[0059] Example 2. Dosing regimen and parameter measurement
[0060] Sorafenib (Bayer, GER) was dissolved in a 1:1 formulation of castor oil and ethanol, diluted with saline (final working concentration was 4 mg / ml, and the intragastric volume was about 50 μl / mouse).
[0061] R848 (Selleck, USA) was dissolved in ethanol and diluted with saline (final working concentration was 0.2 mg / ml, injection volume was 100 μl / mouse).
[0062] The mice in the Sorafenib group were given Sorafenib alone, administered orally, at a dose of 10 mg / kg mouse body weight, once a day, 5 times a week, for a total of 21 days.
[0063] Mice in the R848 group were given R848 alone, intratumoral injection at a dose of 20 μg / mouse, twice a week for 21 days in total.
[0064] The mice in the combination drug group were given Sorafenib and R848 as above, the administration dose was the same as above, and the administration frequency was the same as that of each single drug group, that is, Sorafenib was once...
Embodiment 3
[0068] Example 3. Results Evaluation
[0069] 3.1 Tumor volume and tumor weight in mice
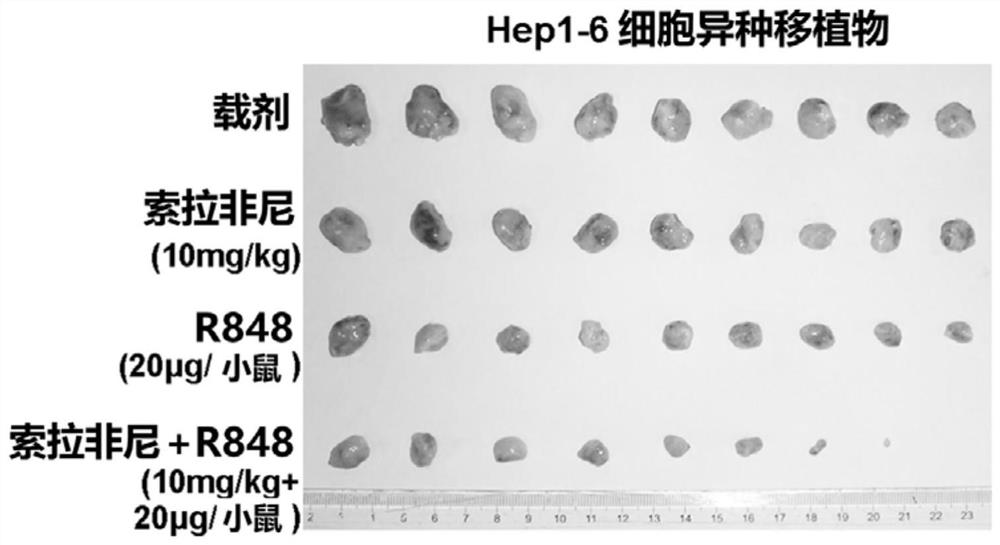
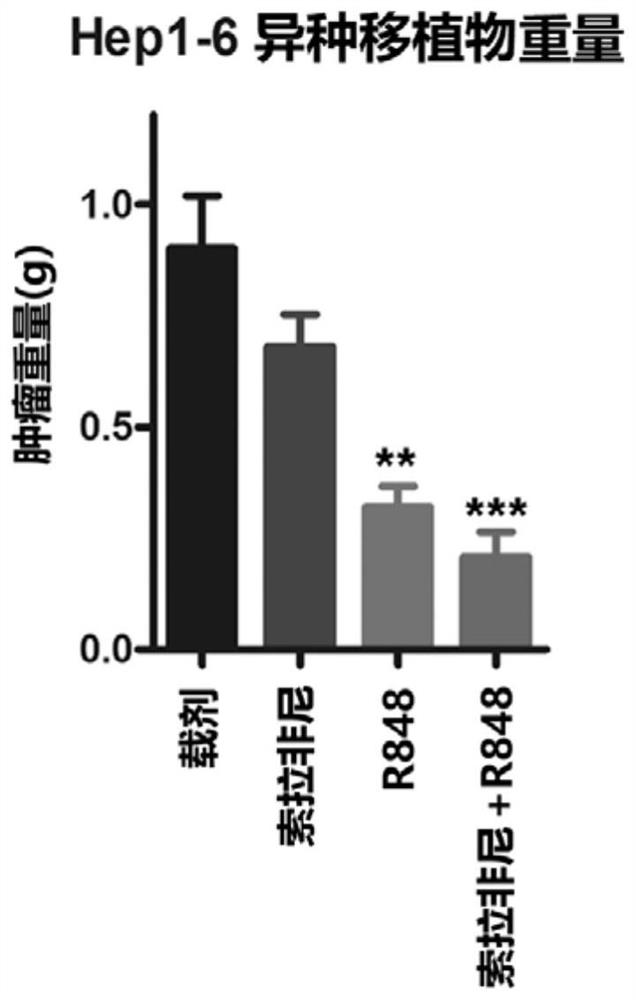
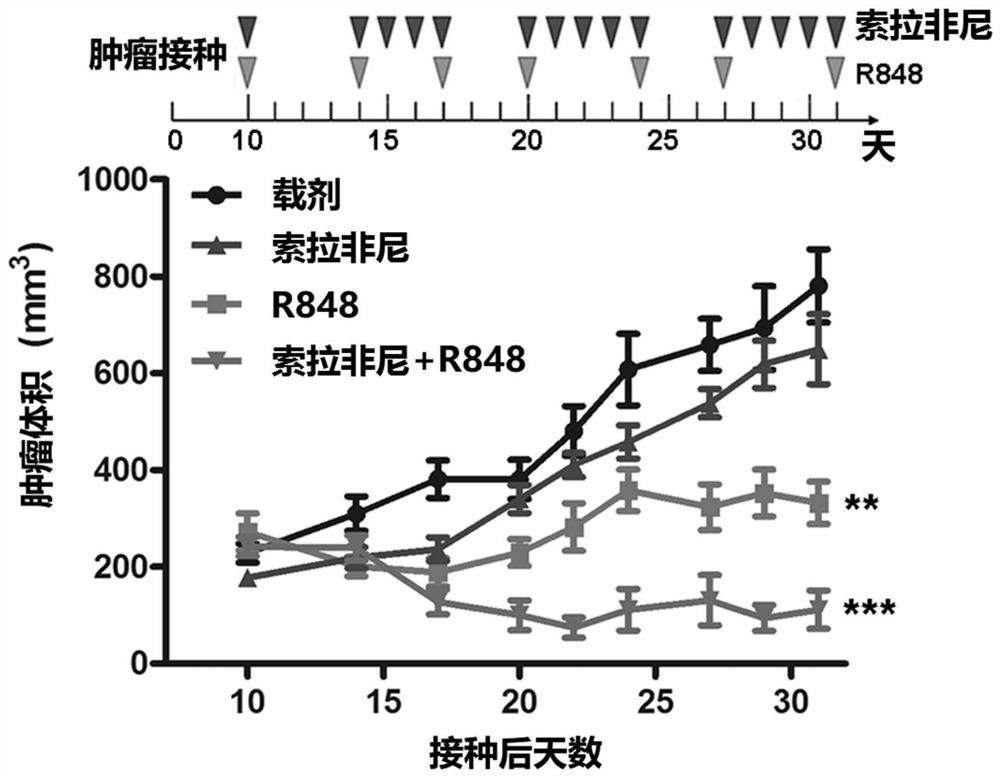
[0070] At the end of the 21-day treatment, mice in each group remained alive. Such as Figure 1A to Figure 1C As shown, R848 alone showed a significant tumor reduction effect after the end of treatment. Since the dose of Sorafenib (about 200 μg / mouse in a single dose) is much higher than that of R848, R848 appears to be more potent than Sorafenib. In addition, compared with single administration of Sorafenib (10mg / kg) or R848 (20μg / mouse), the combination regimen significantly inhibited the tumor volume ( Figure 1A and Figure 1C ) and tumor weight ( Figure 1B ).
[0071] In particular, if Figure 1B As shown in Table 1, compared with the vehicle control, Sorafenib alone reduced the tumor weight to about 76%, but it has not yet reached a statistically significant level; R848 alone reduced the tumor weight to about 36%, with statistical However, the combined drug group reduced the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com