A tumor-targeted gadolinium-based magnetic resonance imaging contrast agent and its preparation method
A contrast agent and gadolinium-based technology, applied in the field of contrast agents, can solve problems affecting clinical application, short residence time, increased injection dose, etc., and achieve good biocompatibility, good biocompatibility, and reduced dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
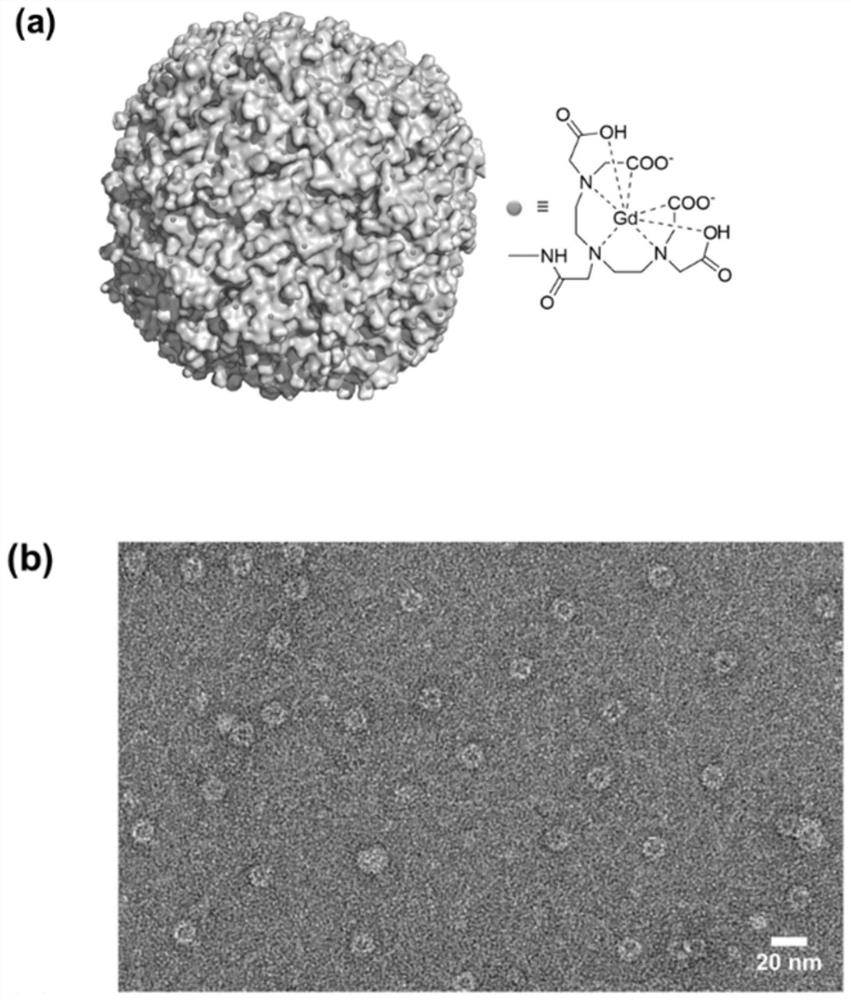
[0037] The preparation of embodiment 1, HFn-Gd
[0038] Using the full-length cDNA of human H subunit ferritin (HFn) as the amplification template, the HFn gene was obtained by polymerase chain reaction, and constructed on the pET11b plasmid, and then the constructed complete plasmid was transformed into Escherichia coli engineering bacteria BL21 , by culturing bacteria, inducing expression, disrupting bacteria, and separating and purifying to obtain recombinant HFn protein, the amino acid sequence of which is shown in SEQ ID NO:1.
[0039] Prepare DTPA and Gd ion solution with the same molar mass, stir and mix well to form the gadolinium chelate Gd-DTPA, use HFn and gadolinium chelate to condense the gadolinium chelate through the amide condensation reaction of the carboxyl group of the chelating agent and the amino group of the protein Connected to the surface of HFn, in the presence of condensing agents EDC and NHS, mix the gadolinium chelate with HFn, so that DTPA and HFn ...
Embodiment 2
[0040] The inspection of embodiment 2, HFn-Gd stability
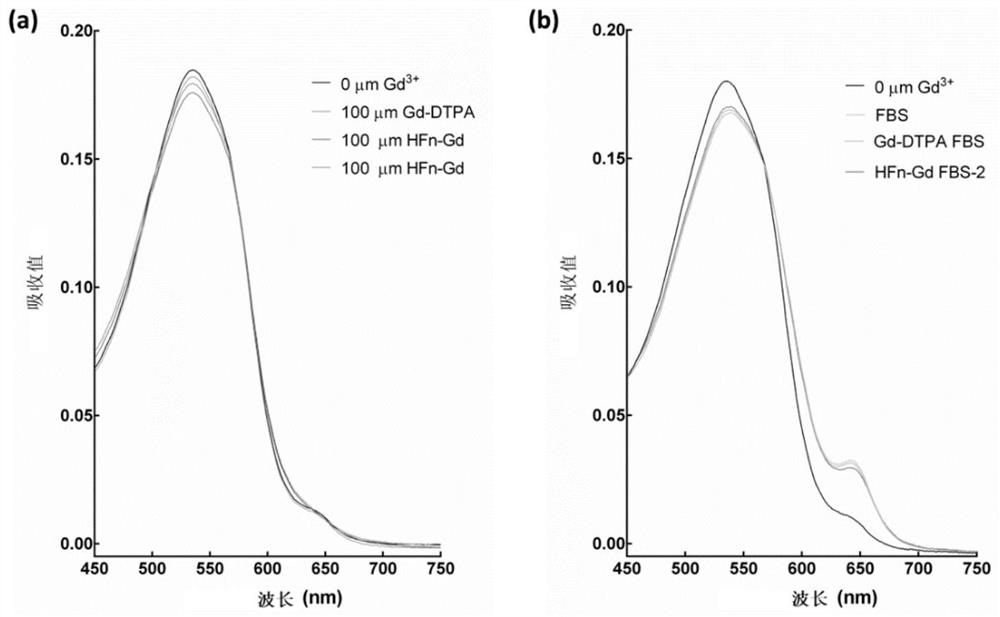
[0041] In order to test the stability of HFn-Gd and confirm that there is no free Gd ion in the solution and serum, add the sample and arsenazoШ solution together, the free metal ion will combine with the solution and form an absorption peak at 650 nanometers, by Judging whether the arsenazoIII solution has an absorption peak at 650 nm indicates whether there is free Gd in the sample. figure 2 a shows the arsenazoIII solution (black line), arsenazoIII added commercial Gd-DTPA (blue line), freshly prepared HFn-Gd (green line) and HFn-Gd (red line) stored for two months The UV / Vis spectral absorption figure of sample, all samples have no absorption peak at 650 nanometers, have shown the stability of HFn-Gd preparation. figure 2 b shows the arsenazoIII solution (black line), arsenazoIII added fetal bovine serum (FBS, blue line), pre-incubated with FBS and commercial Gd-DTPA (red line) and pre-incubated with FBS and HFn-...
Embodiment 3
[0042] The longitudinal relaxation rate (r of embodiment 3, HFn-Gd and commercial Gd-DTPA 1 ) determination
[0043] The HFn-Gd material was first diluted to a final Gd concentration of 0-1 mM, and then assayed in an MRI system (Bruker, BioSpec70 / 20USR). Material T 1 The value is determined using a multi-slice-multi-echo sequence for T 1 Weighted imaging, the specific parameters are field of view (FOV) = 4cm × 4cm, matrix = 256 × 256, repetitiontime (TR) = 150ms, and TE = 1.21 ms (T 1 ). T of different concentrations of HFn-Gd 1 The reciprocal of the value and its concentration value are linearly fitted to obtain the r of the material 1 value. image 3 a is the measured r of different batches of HFn-Gd materials under a magnetic resonance instrument with a field strength of 7T 1 value, an average of 4.78mM -1 the s -1 , image 3 b for its T 1 Magnetic resonance imaging image, the greater the concentration, the lower the signal value and the brighter the image. ima...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com