Production formula and technology of high-treatment-effect cyproheptadine hydrochloride tablets
A technology of cyproheptadine hydrochloride tablets and cyproheptadine hydrochloride, which is applied in the medical field, can solve problems such as adverse reactions, user poisoning, and excessive blood drug concentration, and achieve the effects of simplifying the production process, ensuring safety, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
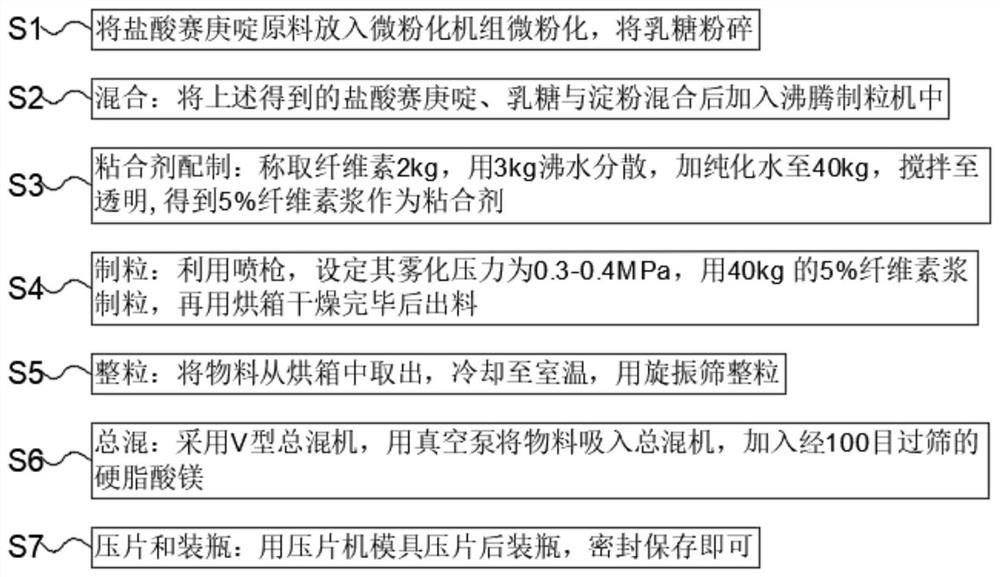
Embodiment
[0038] Embodiment: According to relevant bioequivalence test regulations, carry out human body bioavailability and bioequivalence under fasting and postprandial state with the cyproheptadine hydrochloride tablet produced by Nichi-Iko Pharmaceutical Co., Ltd. Sexual test, while observing the safety of the test preparation and reference preparation in healthy subjects, so as to prove that its quality and curative effect have reached the advanced level abroad. The test results show that: the pharmacokinetic parameters AUC and Cmax are analyzed by the mixed effect model of double crossover design after logarithmic transformation, and double one-sided test (TOST), the geometric mean ratio of test preparation and reference preparation AUC If the 90% confidence interval falls within the range of 80.00% to 125.00%, and the 90% confidence interval of the Cmax geometric mean ratio falls within the range of 80.00% to 125.00%, the test preparation and the reference preparation are bioequiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com