Preparation method of hydroxychloroquine
A technology of hydroxychloroquine and hydroxyquinoline, which is applied in the field of preparation of hydroxychloroquine, can solve the problems affecting the final quality of the product, unfavorable industrial application, high cost, etc., and is beneficial to large-scale production, prevents the generation of isomers, and is not easy to produce. The effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
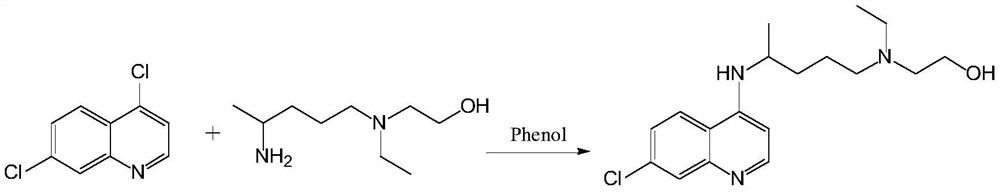
[0066] In the deep eutectic solvent (200g) that choline chloride and urea (molar ratio 1:2) form, add 7-chloro-4-hydroxyquinoline p-toluenesulfonate (333.0g, 1.0mol) and Hydroxychloroquine side chain (182.7g, 1.05mol) was subjected to a constant temperature reaction at 80° C. for 9 hours, and TLC detected that the reaction was complete.
[0067] Cool down to 25°C, add ethyl acetate (300 mL), add cold water (500 mL) while fully stirring, let stand for 2 hours, separate the ethyl acetate solution, wash with water, and concentrate to obtain a crude product. Isopropanol was recrystallized to obtain off-white crystals (289 g), with a yield of 86%. Melting point: 91-92°C.
[0068] The content of hydroxychloroquine analyzed by liquid chromatography was 99.54%.
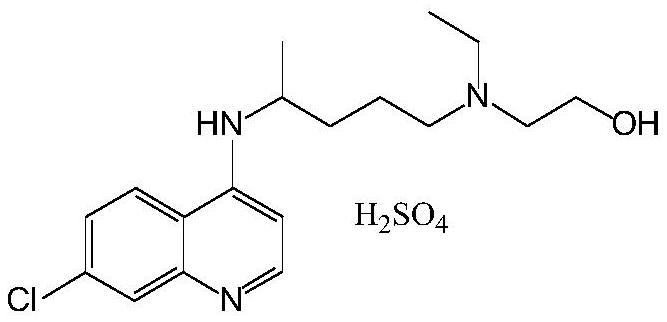
[0069] The preparation of hydroxychloroquine sulfate
[0070] Under ice-water cooling, the concentrated sulfuric acid (20g) is slowly added in the solution of isopropanol (80g), and is slowly dropped in the solution of hyd...
Embodiment 2
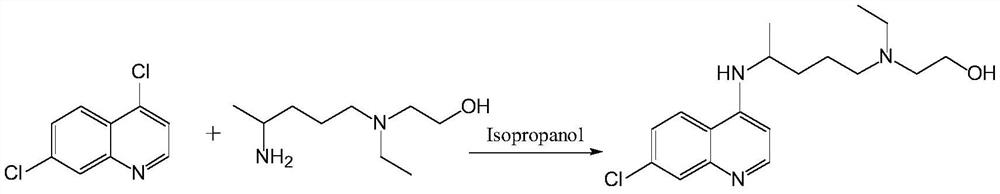
[0073] In the deep eutectic solvent (50g) that choline chloride and urea (molar ratio 1:2) form, add 7-chloro-4-hydroxyquinoline p-toluenesulfonate (33.3g, 0.1mol) and Hydroxychloroquine side chain (17.4 g, 0.1 mol) was reacted at a constant temperature of 85° C. for 8 hours, and the reaction was detected by TLC.
[0074] Cool down to 25°C, add ethyl acetate (50 mL), add cold water (100 mL) while fully stirring, let stand for 2 hours, separate the ethyl acetate solution, wash with water, and concentrate to obtain a crude product. Isopropanol was recrystallized to obtain off-white crystals (30 g) with a yield of 91%. Melting point: 91-92°C.
[0075] The content of hydroxychloroquine analyzed by liquid chromatography was 99.62%.
[0076] The preparation of hydroxychloroquine sulfate
[0077] According to the preparation process described in Example 1, hydroxychloroquine sulfate was obtained, with a melting point of 239° C. to 241° C.
[0078] HPLC purity 99.79%, the largest s...
Embodiment 3
[0080] In the deep eutectic solvent (50g) that choline chloride and urea (molar ratio 1:2) are formed, add 7-chloro-4-hydroxyquinoline trifluoromethanesulfonate (32.7g, 0.1mol) and hydroxyl Chloroquine side chains (20.9 g, 0.12 mol) were subjected to a constant temperature reaction at 70° C. for 5 hours, and TLC detected that the reaction was complete.
[0081] Cool down to 25°C, add ethyl acetate (50 mL), add cold water (100 mL) while fully stirring, let stand for 2 hours, separate the ethyl acetate solution, wash with water, and concentrate to obtain a crude product. Isopropanol was recrystallized to obtain off-white crystals (32.3 g), with a yield of 96%. Melting point: 91-92°C.
[0082] The content of hydroxychloroquine analyzed by liquid chromatography was 99.69%.
[0083] The preparation of hydroxychloroquine sulfate
[0084] According to the preparation process described in Example 1, hydroxychloroquine sulfate was obtained, with a melting point of 239° C. to 241° C. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com