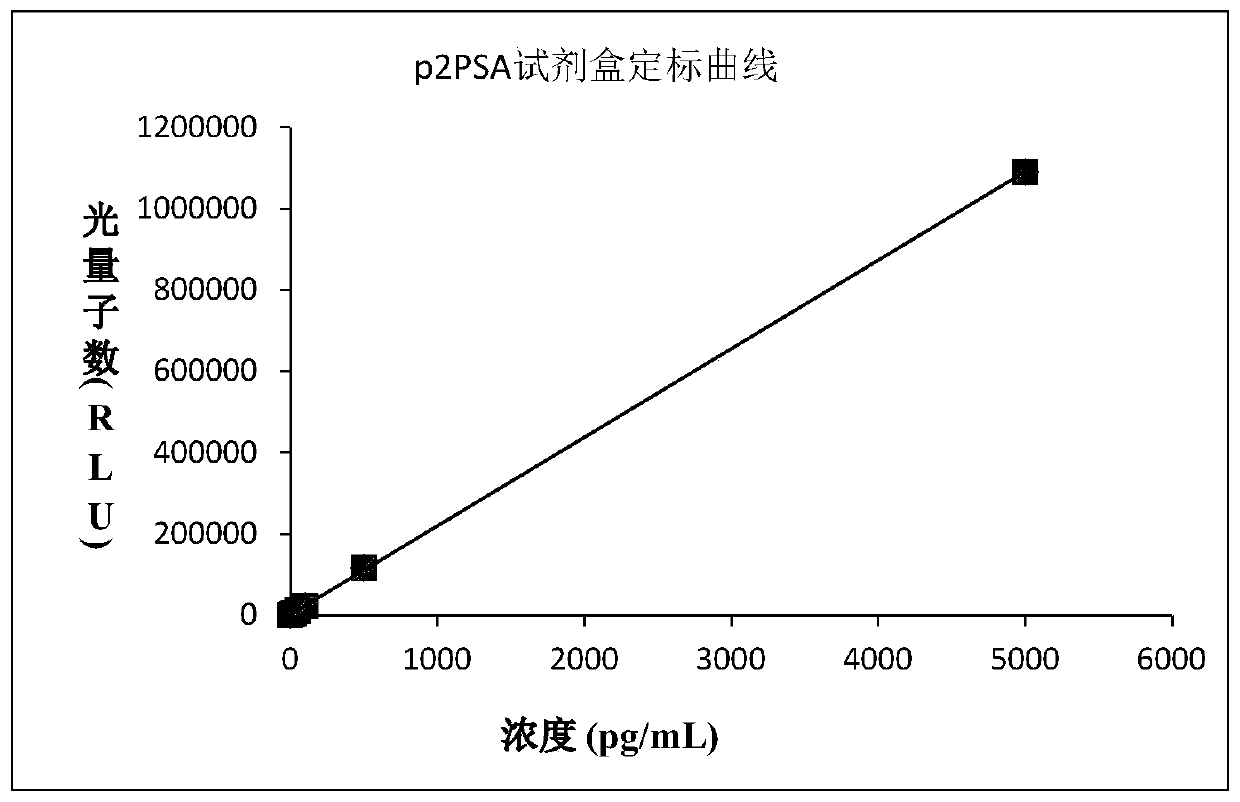
Prostate specific antigen homoisomer chemiluminescence immunoassay kit and preparation method thereof
A chemiluminescent immune and prostate-specific technology, which is applied in the direction of measuring devices, scientific instruments, instruments, etc., can solve the problems of unfavorable popularization, high price, and large economic burden of patients, so as to save time and cost, low cost, and reduce manual operations effect of error
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention also provides a preparation method of a prostate-specific antigen isomer (p2PSA) chemiluminescent immunoassay kit, comprising the following steps:
[0050] Step 1: Preparation of magnetic particle reaction solution R1
[0051] Mix buffer and preservative to prepare R1 basic buffer;
[0052] Take commercially available streptavidin magnetic beads (selected from JSR Company, model MS300 / Streptavidin), the magnetic beads have a particle size of 3 μm, wash with PBS (20mmol / L PB and 150mmol / L NaCl) buffer solution with pH 7.2 Three times, then resuspend the magnetic beads into the R1 basic buffer to prepare the magnetic particle reaction solution R1 with a mass concentration of 0.03%-0.1%;
[0053] Step 2: Preparation of tracer conjugate reaction solution R2
[0054] Mix NaCl, buffer, preservative, blocking agent, protein stabilizer and surfactant to prepare R2 basic buffer;
[0055] Take the p2PSA antibody (or tPSA antibody), and label it with the a...
Embodiment 1
[0069] 1.1 Kit composition
[0070]
[0071]
[0072] 1.2 Preparation method of the kit
[0073] (1) Preparation of magnetic particle reaction solution R1
[0074] Take streptavidin magnetic beads with a particle size of 3 μm, wash them three times with PBS buffer at pH 7.2, then resuspend the magnetic beads into an appropriate amount of R1 basic buffer (other components except magnetic beads), and prepare Prepare a magnetic particle reaction solution R1 with a mass concentration of 0.1%.
[0075] (2) Preparation of tracer conjugate reaction solution R2
[0076] a. Preparation of acridinium ester-labeled p2PSA antibody:
[0077] Take an appropriate amount of p2PSA antibody, and label it with acridinium ester (3mg / mL) dissolved in DMF according to the ratio of molar concentration 1:7.5. The labeling buffer is pH7.4PB (20mmol / L), and the labeling time is 4h. The reaction process requires Avoid light. After the labeling reaction, use AKTA purification instrument (G25 g...
Embodiment 2
[0088] 1.1 Kit composition
[0089]
[0090]
[0091] 1.2 Preparation method of the kit
[0092] (1) Preparation of magnetic particle reaction solution R1
[0093] Take streptavidin magnetic beads with a particle size of 3 μm, wash them three times with PBS buffer at pH 7.2, then resuspend the magnetic beads into an appropriate amount of R1 basic buffer (other components except magnetic beads), and prepare Prepare a magnetic particle reaction solution R1 with a mass concentration of 0.1%.
[0094] (2) Preparation of tracer conjugate reaction solution R2
[0095] a. Preparation of acridinium ester-labeled tPSA antibody:
[0096] Take an appropriate amount of tPSA antibody and label it with acridinium ester (3mg / mL) dissolved in DMF at a molar concentration ratio of 1:3. The labeling buffer is pH7.4PB (20mmol / L), and the labeling time is 12h. The reaction process requires Avoid light. After the labeling reaction, use AKTA purification instrument (G25 gel column) to pu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com