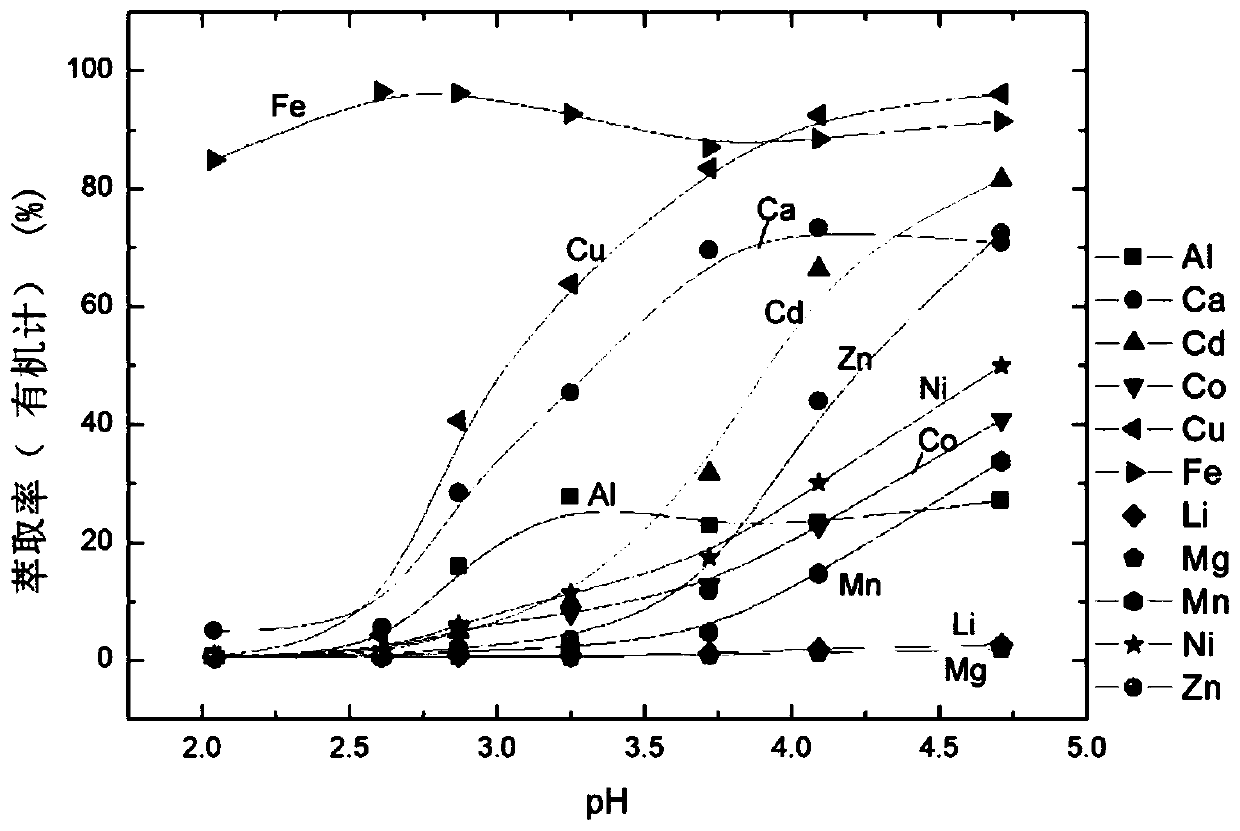
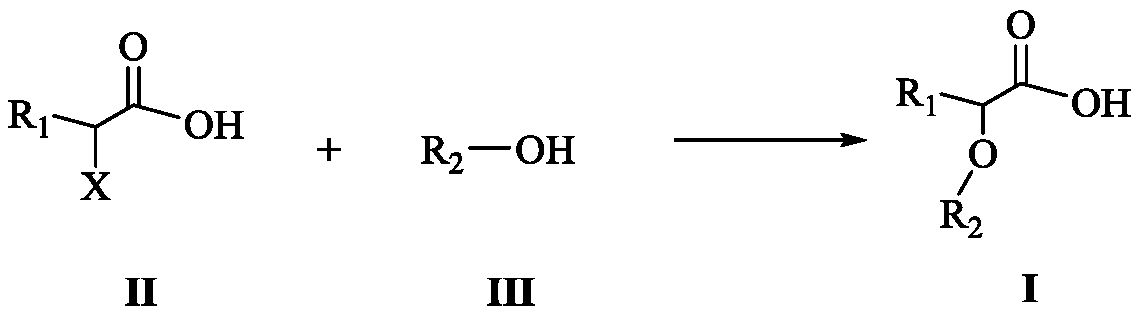
Carboxylic acid compound as well as preparation method and application thereof
A compound and carboxylic acid technology, applied in the field of carboxylic acid compounds, can solve the problems of complex operation procedures, environmental pollution, and high process costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081]
[0082] Add 50g of isooctyl alcohol, 225mL of tetrahydrofuran (THF), and 8.8g of sodium particles into a three-necked flask, react at 60-70°C for 6 hours, a large amount of white solids are formed, and a small amount of sodium particles remain; L 2-bromooctanoic acid THF solution and continue to react at 60°C for 4h; after cooling, remove THF by rotary evaporation, add 200mL water and 200mL ethyl acetate (EA) to the concentrated solution, shake and separate, take the water layer; Acidify with hydrochloric acid to pH ≈ 1, extract with ethyl acetate, wash the organic phase twice with water, and spin dry to obtain 65 g of a light yellow product, namely compound BC195. 1 H NMR (400MHz, CDCl 3 )δ4.1(1H), 3.52(1H), 3.35(1H), 1.82(2H), 1.54(3H), 1.20-1.31(14H), 0.91(6H), 0.87(3H); 13 C NMR (101MHz, CDCl 3 )δ171(s), 79(s), 72(s), 36(s), 32(s), 29(s), 26-28(m), 22–23(m), 14(s), 11(s); MS[M-H] - :271.
Embodiment 2
[0084]
[0085] Add 28.6g of isooctyl alcohol, 200mL of tetrahydrofuran (THF), 8.8g of 60% sodium hydride (dispersed in mineral oil) into the three-necked flask, and react at 60-70°C for 6 hours, a large amount of white solids are formed, and a small amount of sodium particles remain; Add 20mL of 10mol / L THF solution of 2-bromohexanoic acid dropwise at 60°C and continue to react at 60°C for 4h; after cooling, remove THF by rotary evaporation, add 200mL of water and 200mL of ethyl acetate (EA) to the concentrated solution, shake The layers were separated, and the water layer was taken; the water layer was acidified with hydrochloric acid to pH ≈ 1, extracted with ethyl acetate, the organic phase was washed twice with water, and spin-dried to obtain 38 g of a light yellow product, namely compound BC196. 1 H NMR (400MHz, CDCl 3 )δ3.97(1H), 3.41(1H), 3.26(1H), 1.70(2H), 1.45(3H), 1.05-1.24(10H), 0.91(9H); 13 C NMR (101MHz, CDCl3) δ175(s), 82(s), 76(s), 40(s), 32(s), 30(s), 29(...
Embodiment 3
[0087]
[0088] Add 32g of n-octanol, 200mL of tetrahydrofuran (THF), and 5.7g of sodium particles into a three-necked flask, react at 60-70°C for 6 hours, a large amount of white solids are formed, and a small amount of sodium particles remain; L of THF solution of 2-bromohexanoic acid and continue to react at 60°C for 4 hours; after cooling, remove THF by rotary evaporation, add 200mL water and 200mL ethyl acetate (EA) to the concentrated solution, shake and separate, and take the water layer; the water layer Acidify with hydrochloric acid to pH ≈ 1, extract with ethyl acetate, wash the organic phase twice with water, and spin dry to obtain the target compound, compound BC191.
[0089] Compound BC191 1 H NMR (400MHz, CDCl 3 )δ12.53(1H), 4.01(1H), 3.32(2H), 1.65(2H), 1.20-1.32(16H), 0.89(6H); 13 C NMR (101MHz, CDCl 3 )δ173(s), 81(s), 65(s), 32-30(m), 22–23(m), 14(s); MS[M-H] - :243.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com