Design and synthesis of a carbazole-based fluorescent derivative reagent and its application in the detection of fipronil and fipronil metabolites
A derivative reagent, carbazole-based technology, applied in the design and synthesis of carbazole-based fluorescent derivative reagents, the application field of detection of fipronil and fipronil metabolites, can solve the problem of complex pre-treatment of samples and poor sensitivity , high cost of testing, etc., to achieve the effect of fipronil and its metabolites pre-column derivatization detection method with sensitivity, good application prospects, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
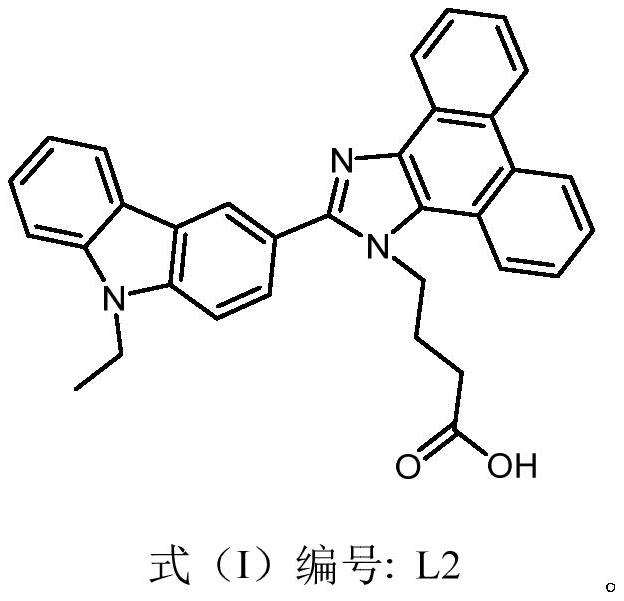
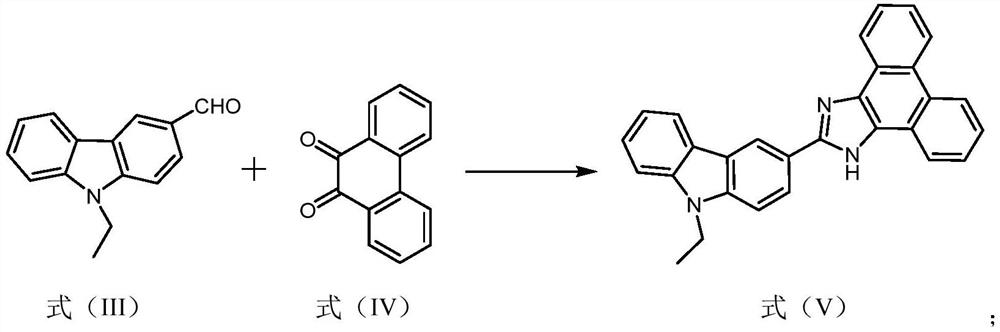
[0034] Example 1: Carbazole-based fluorescent derivatization reagent L2 (4-(2-(9-ethyl-9H-carbazol-3-yl)-1H-phenanthro[9,10-d] with the structure of formula (I) ]imidazol-1-yl) butanoic acid) preparation
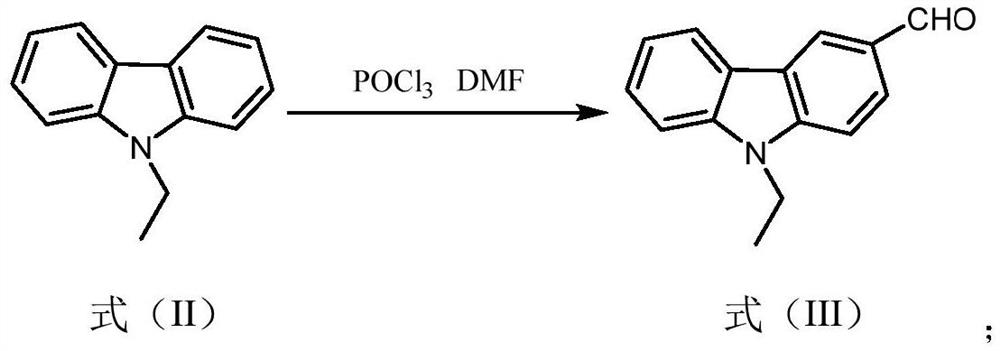
[0035] (1) Preparation of intermediates with formula (Ⅲ) structure
[0036] Weigh 9-ethylcarbazole (5.86g, 30mmol) and dissolve it in 50mL of DMF solution, add phosphorus oxychloride (7mL, 75mmol) dropwise under ice-cooling at 0°C, and complete the dropwise addition within 30min. Then the temperature was raised to 98°C, stirred under reflux, and the progress of the reaction was detected by TLC. After the reaction was finished, cool to room temperature, dilute with 150 mL of water, extract with dichloromethane (3×100 mL), combine the organic phases, dry over anhydrous sodium sulfate, precipitate from the solvent, mix the sample with silica gel, and purify and separate by column chromatography (the eluent is Petroleum ether: ethyl acetate=15:1, v / v), the intermediate compoun...
Embodiment 2
[0043] Example 2: Establishment of a method for detecting fipronil by pre-column derivatization of a carbazole-based fluorescent derivatization reagent with a structure of formula (I)
[0044] (1) Preparation of fipronil pre-column derivatization reaction product
[0045] The pre-column derivatization reaction of fipronil is shown in the figure below:
[0046]
[0047] The fluorescent derivatization reagent L2 (0.16g, 1mmol) and dichloromethane (40mL) were added to a 100mL eggplant-shaped flask, and DMAP (0.12g, 3mmol), DCC (0.23g, 1.5mmol) and fluorine were added sequentially under stirring at 35°C. Chrynil (0.07g, 0.5mmol), continue to stir the reaction, TLC detection of the reaction progress. After the reaction, wash twice with 20mL of saturated brine and once with 20mL of water, extract the water layer with dichloromethane (3×50mL), dry over anhydrous sodium sulfate, remove solvent, and purify and separate by silica gel column chromatography (elution The solvent is n-...
Embodiment 3
[0053] Example 3: Establishment of a method for detecting fipronil metabolites by pre-column derivatization of a carbazole-based fluorescent derivatization reagent with a structure of formula (I)
[0054] (1) Pre-column derivatization reaction of fipronil metabolite (fipronil sulfoxide)
[0055] The main conditions for the pre-column derivatization of fipronil metabolites (fipronil sulfoxide) are as follows: EDC / DMAP is used as a catalytic condensation agent, dichloromethane is used as a solvent, and the molar concentration ratio of fipronil sulfoxide to L2 is 1: 8. Reaction in a water bath at 45°C for 75 minutes, the pre-column derivatization reaction is complete, and the derivative product is N-(3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4 -((trifluoromethyl)thio)-1H-pyrazol-5-yl)-4-(2-(9-ethyl-9H-carbazol-3-yl)-1H-phenanthro[9, 10-d] imidazol-1-yl) butanamide (DX1), which has strong fluorescence intensity.
[0056] (2) Pre-column derivatization of fipronil metabol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com