Carboxylic acid derivatization reagent as well as preparation method and application thereof
A technology for derivatizing reagents and carboxylic acids, applied in the field of analytical chemistry, can solve the problems of insufficient sensitivity, complicated derivatization operations, and the influence of strong ionization efficiency of derivatization reagents, and achieves high reactivity, mild derivatization conditions, and properties. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] In another specific embodiment of the present invention, the synthesis method includes the following steps: Dissolve 1-(2-dimethylaminoethyl)piperazine in diethyl ether at room temperature, then add methyl iodide, and stir to obtain .
[0034] In another specific embodiment of the present invention, the molar feed ratio of 1-(2-dimethylaminoethyl)piperazine to methyl iodide in the above method may be 3-10:1, preferably 5:1.
[0035] In another specific embodiment of the present invention, the synthesis method further includes: filtering the reaction liquid after the reaction, washing with ether, and drying to obtain a solid powder, that is, to obtain N,N,N-trimethyl-2-(piperazine- 1-yl)ethane-1-ammonium iodide.
[0036] In another specific embodiment of the present invention, the application of the above-mentioned compound as a derivatization reagent is provided; specifically, the derivatization reagent is a carboxylic acid derivatization reagent; more specifically, the appli...
Embodiment 1
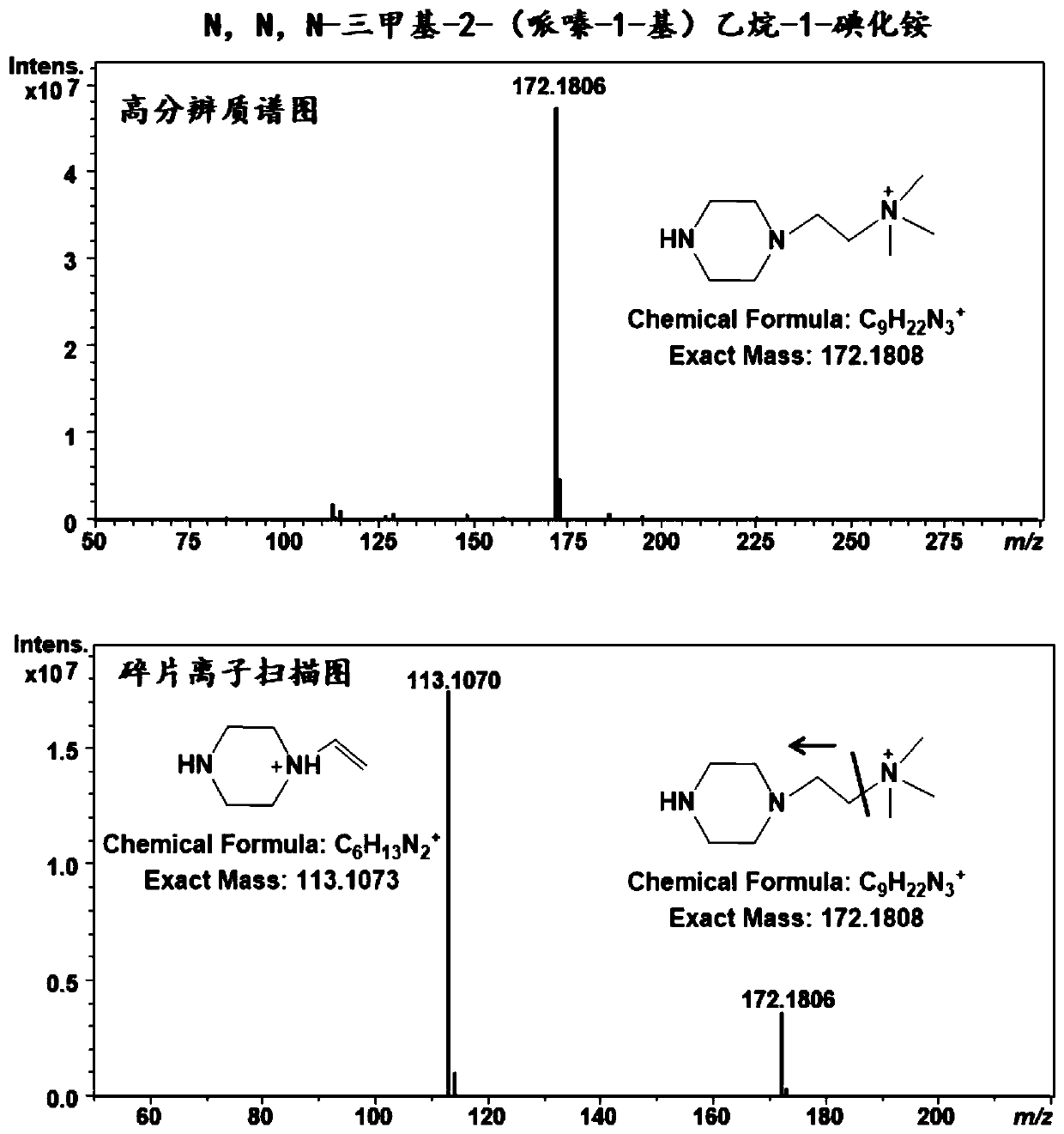
[0051] Example 1: Synthesis of N,N,N-trimethyl-2-(piperazin-1-yl)ethane-1-ammonium iodide
[0052] (1) Add 2.5 mM 1-(2-dimethylaminoethyl)piperazine to 20 mL of ether and vortex for 3 minutes;
[0053] (2) Add 0.5 mM methyl iodide to the above solution and vortex for 3 minutes;
[0054] (3) At room temperature, stir the above solution under a magnetic stirrer at 100rpm / min for 1 hour, then filter it, wash it with ether 3 times, and dry the solid matter on the filter paper to obtain N, N, N- Trimethyl-2-(piperazin-1-yl)ethane-1-ammonium iodide.
[0055] figure 1 It is the high resolution mass spectrum and product ion scanning mass spectrum of N,N,N-trimethyl-2-(piperazin-1-yl)ethane-1-ammonium iodide.
Embodiment 2
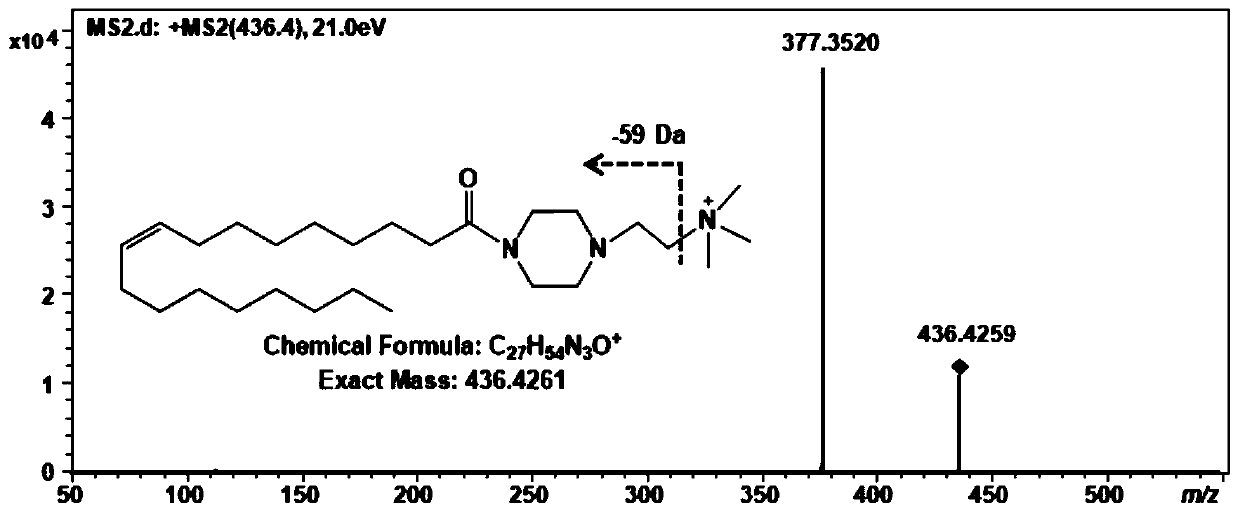
[0056] Example 2: Derivatization reaction and detection of carboxylic acid metabolites in solution
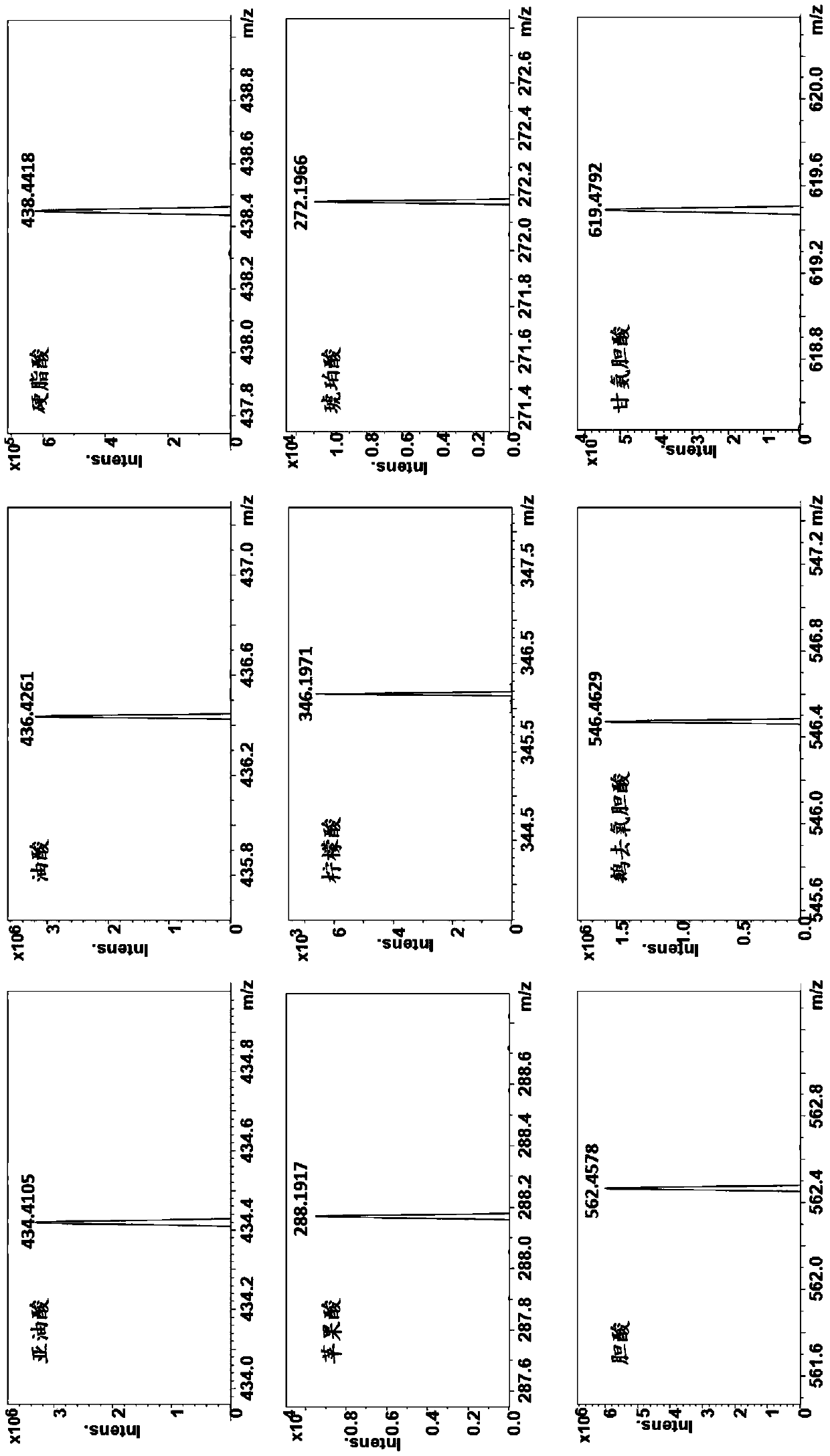
[0057] (1) Precisely weigh 10 mg of cholic acid, oleic acid, and fumaric acid in a 10 mL volumetric flask, add 10 mL of acetonitrile-water (9:1, v / v) solution, vortex to mix, and ultrasound for 10 minutes to obtain 1.0 mg / mL of oleic acid, malic acid, and cholic acid solution for use;
[0058] (2) Precisely weigh 10mg of N,N,N-trimethyl-2-(piperazin-1-yl)ethane-1-ammonium iodide derivatization reagent and place it in a 10mL volumetric flask, add 10mL acetonitrile-water (9:1, v / v) solution, vortex to mix, and ultrasonic for 10 minutes to obtain 1.0 mg / mL of N, N, N-trimethyl-2-(piperazin-1-yl)ethane-1 -Ammonium iodide solution for use;
[0059] (3) Precisely weigh 5 mg of HATU in a 10 mL volumetric flask, add 10 mL of acetonitrile-water (9:1, v / v) solution, vortex to mix, and ultrasound for 10 minutes, for use;
[0060] (4) Precisely weigh 10mg of N,N-dimethylethylenediamine in a 10m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com