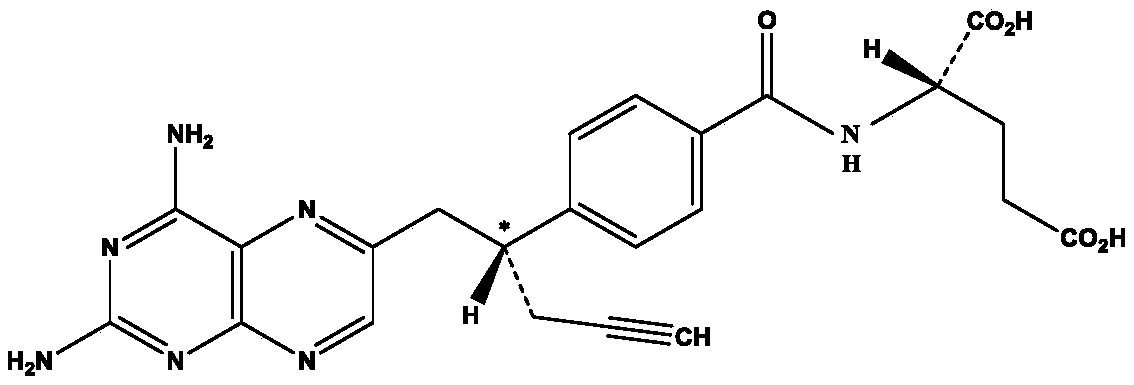
Pralatrexate injection as well as preparation method and application thereof
A technology of pralatrexate injection and pralatrexate, which is applied in the field of pralatrexate injection and its preparation, can solve the problems of inconvenient production, transportation and use, and achieve a wide range of applicable people, low production cost, good safety and stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
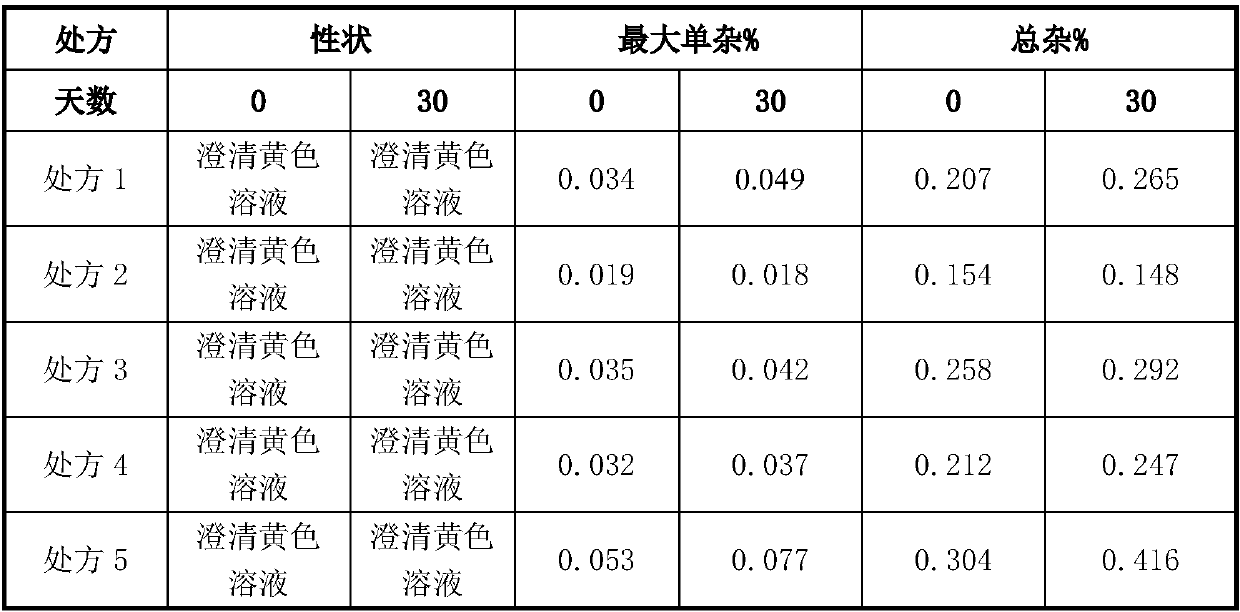
[0043] components Prescription 1 Prescription 2 Prescription 3 Prescription 4 Prescription 5 Pralatrexate 2g 2g 2g 2g 2g Sodium chloride 0.9g 0.9g 0.9g 0.9g 0.9g stabilizer Hydrochloric acid 0.4g Lactic acid 0.4g Acetic acid 0.4g Citric acid 0.4g Phosphoric acid 0.4g sodium hydroxide Adjust pH6.8 Adjust pH6.8 Adjust pH6.8 Adjust pH6.8 Adjust pH6.8 Water for Injection Add to 100mL Add to 100mL Add to 100mL Add to 100mL Add to 100mL
[0044] Prepare prescriptions according to the above table and store them for 30 days at 25°C±2°C / 60%RH±5%RH. The results are as follows:
[0045]
[0046] According to the results, it can be seen that when lactic acid is selected as the stabilizer, the content of related substances is lower.
Embodiment 1
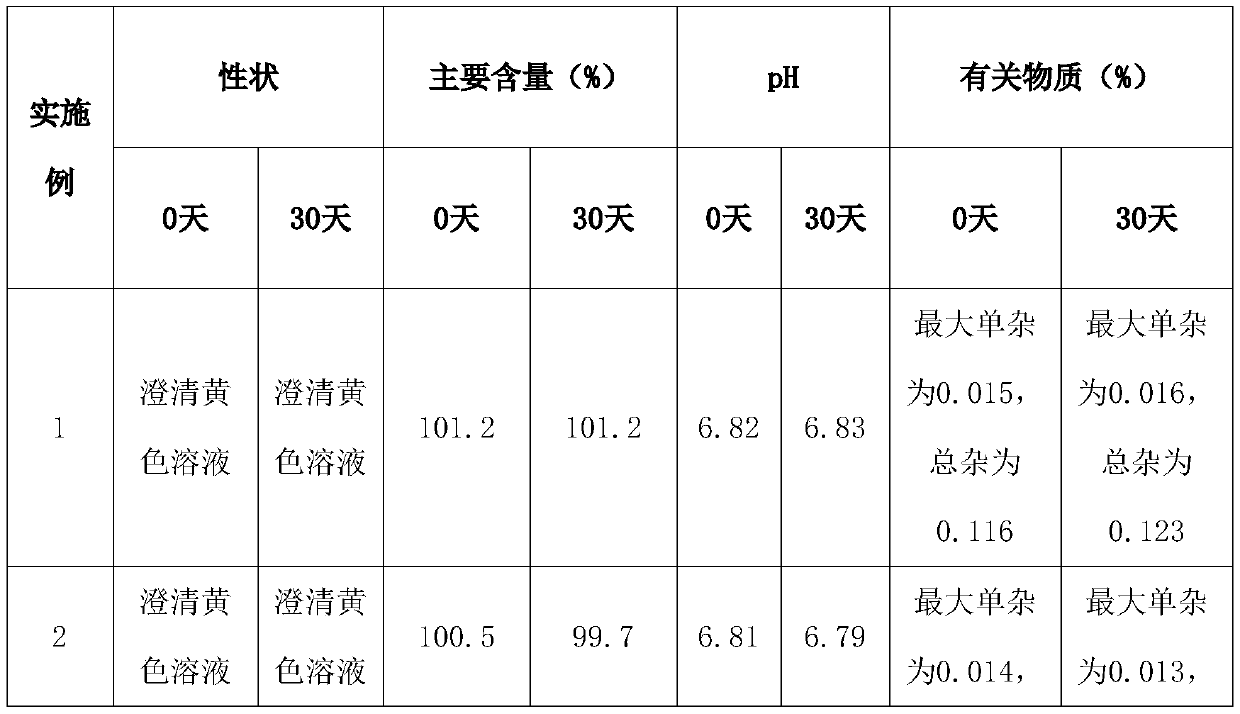
[0049] A pralatrexate injection. The injection is composed of: 2 g of pralatrexate, 4 g of sorbitol, 0.5 g of lactic acid, an appropriate amount of sodium hydroxide to adjust the pH to 6.8, and water for injection to 100 mL.
[0050] Take 70%-80% of the target volume of sterile water for injection, add 4g of sorbitol while stirring, add 2g of pralatrexate raw material to the solution, and stir until completely dissolved, add 0.5g of lactic acid, adjust with appropriate amount of sodium hydroxide pH to 6.8, continue stirring. Finally, add sterile water for injection to reach the target volume. After the preparation, the solution was clarified by filtering the solution with a 0.22 μm disposable sterile PVDF membrane filter. After clarification and filtration, the clarified medicinal solution was passed through two sets of hydrophilic 0.22 μm PVDF membrane filters for sterilization. The filtered medicinal solution is filled into sterile vials and sealed to obtain the product. ...
Embodiment 2
[0052] A pralatrexate injection. The injection consists of: 2 g of pralatrexate, 4.5 g of sorbitol, 0.5 g of lactic acid, an appropriate amount of sodium hydroxide to adjust the pH to 6.8, and water for injection to 100 mL.
[0053] Take sterile water for injection with a target volume of 70%-80%, add 4.5g of sorbitol while stirring, add 2g of pralatrexate raw material to the solution, and stir until completely dissolved, add 0.5g of lactic acid and appropriate amount of sodium hydroxide Adjust the pH to 6.8 and continue stirring. Finally, add sterile water for injection to reach the target volume. After the preparation, the solution was clarified by filtering the solution with a 0.22 μm disposable sterile PVDF membrane filter. After clarification and filtration, the clarified medicinal solution was passed through two sets of hydrophilic 0.22 μm PVDF membrane filters for sterilization. The filtered medicinal solution is filled into sterile vials and sealed to obtain the prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com