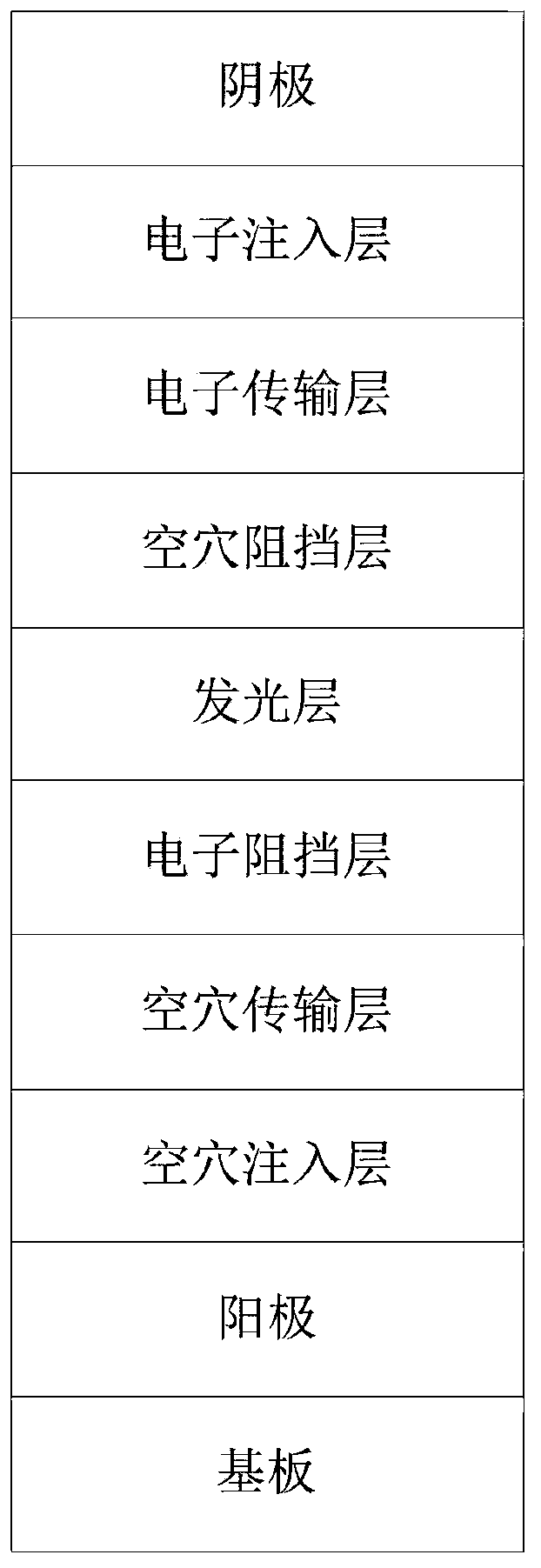
Aza-naphthalene derivative, synthesis method and electronic device thereof
A technology for electronic devices and derivatives is applied in the fields of electronic devices, azanaphthalene derivatives and their synthesis, and achieves the effects of excellent electron transport performance, low driving voltage and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Embodiment 1: the synthesis of compound 1-31
[0123] (Synthesis of intermediate M1)
[0124] The synthetic route of intermediate M1 is as follows:
[0125]
[0126] Add benzohydrazide (2.3g, 20mmol), 2-bromoacetophenone (2.0g, 10mmol), sodium acetate (2.0g, 15mmol) and 120mL mixed solvent (ethanol: acetic acid=7: 3 (V / V)), reflux and stirred for 12 hours. After the reaction was complete, the solid was collected by suction filtration and washed with a small amount of absolute ethanol. The crude product was further purified by column chromatography (petroleum ether:dichloromethane=3:1 (V / V)). The solvent was evaporated, and after drying, 1.3 g of light yellow solid was obtained with a yield of 58%. MS (EI): m / z: 233.58 [M + ]. Anal.calcd for C 15 h 11 N 3 (%): C 77.23, H 4.75, N 18.01; found: C 77.10, H 4.70, N 17.90. (Synthesis of Intermediate M3)
[0127] The synthetic route of intermediate M2 is as follows:
[0128]
[0129] Under an argon atmosphere...
Embodiment 2
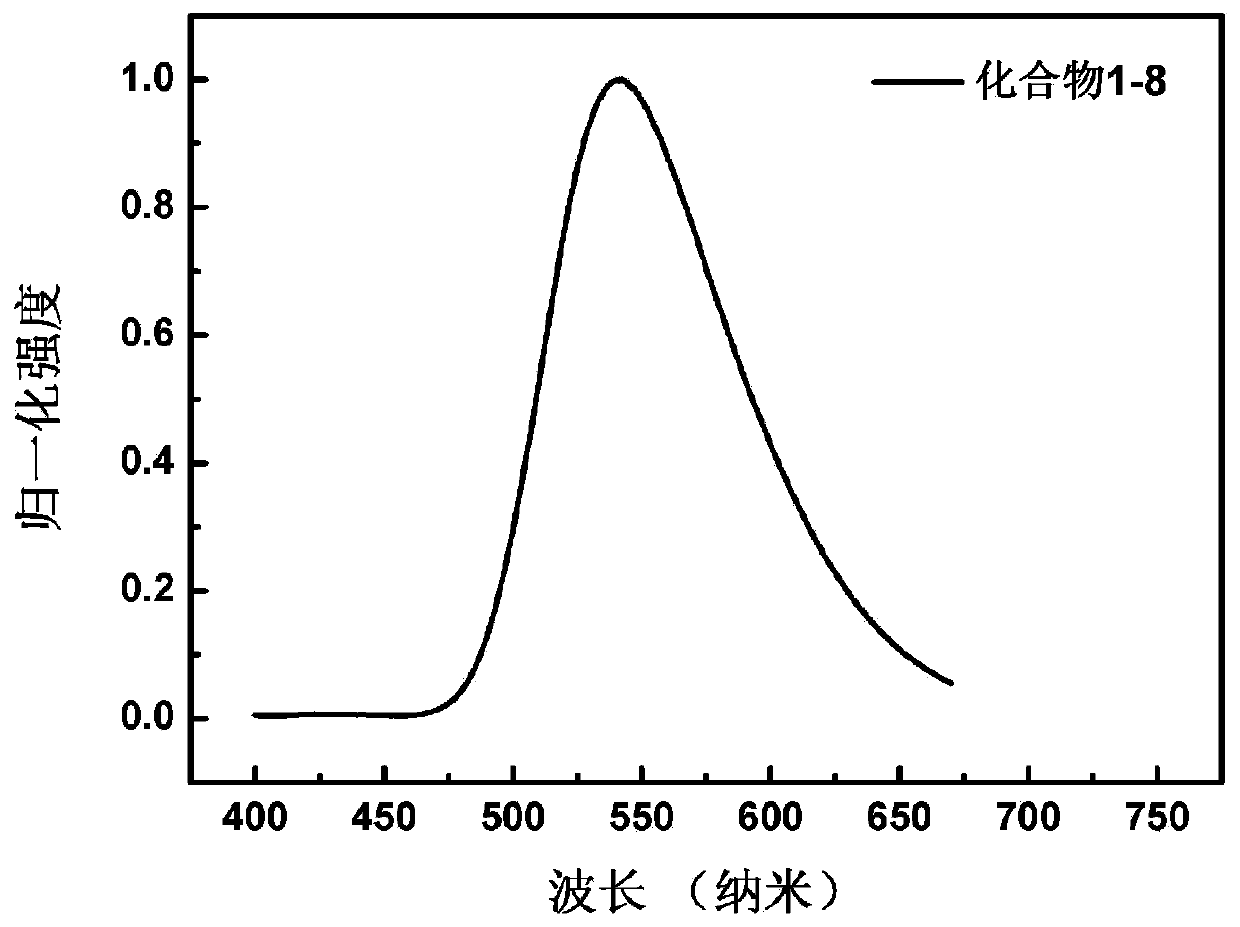
[0134] Embodiment 2: the synthesis of compound 1-8
[0135] (Synthesis of Compounds 1-8)
[0136] Compound 1-8 synthetic route is as follows:
[0137]
[0138] In a 250mL two-necked flask, 7.2g (23.0mmol) M2, 9.6g (29.0mmol) 5,7-dihydro-5-phenylindolo[2,3-B]carbazole, 3.0g (29.0mmol) ) sodium tert-butoxide, 0.1g (0.3mmol) tri-tert-butylphosphine tetrafluoroborate, 0.27g (0.3mmol) tris(dibenzylideneacetone) dipalladium, after degassing the reaction system, under the protection of nitrogen, add 150mL of toluene was stirred and heated to reflux for 12 hours. After the reaction was complete, the system was cooled to room temperature, filtered under reduced pressure, and the filter residue was washed with a large amount of dichloromethane, and the filtrate was concentrated to obtain a crude product, which was eluted with petroleum ether:dichloromethane=2:3 (volume ratio) The reagent was separated and purified on a silica gel column to obtain 13.0 g of a white solid with a yie...
Embodiment 3
[0139] Embodiment 3: the synthesis of compound 1-14
[0140] (Synthesis of Compound 1-14)
[0141] Compound 1-14 synthetic route is as follows:
[0142]
[0143] Under nitrogen, add 13.8g (47.8mmol) triphenylamine 4-borate, 8.4g (79.6mmol) anhydrous sodium carbonate, 12.7g (39.8mmol) M2, 470.8mg (4.8mmol) ) tetrakis(triphenylphosphine palladium) and 100 mL mixed solvent (toluene:water:ethanol=5:1:1 (V / V)). The system was gradually heated to reflux and reacted overnight under reflux. After the reaction was completed, the heating was stopped, and the reaction system was cooled to room temperature by itself. The reaction solution was poured into about 200 mL of water, and extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and further purified by column chromatography (350 mesh silica gel, eluent: petroleum ether: dichloromethane = 3:2 (V / V)) to obtain a red solid 17.7g, yield 84%. MS (EI): m / z: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com