Pyridine formylaryl (heteroaryl) alpha-substituted amino acid compound as well as preparation method and application thereof
A compound and heteroaryl technology, applied in the fields of amino acid compounds substituted at the α-position of picolyl aryl (heteroaryl), its preparation and application, which can solve the problem of insufficient activity and selectivity, and few researches on inhibitors, etc. problems, to achieve the effects of treating and preventing anemia, preventing and alleviating gastrointestinal toxicity and hematopoietic function damage, and promoting expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
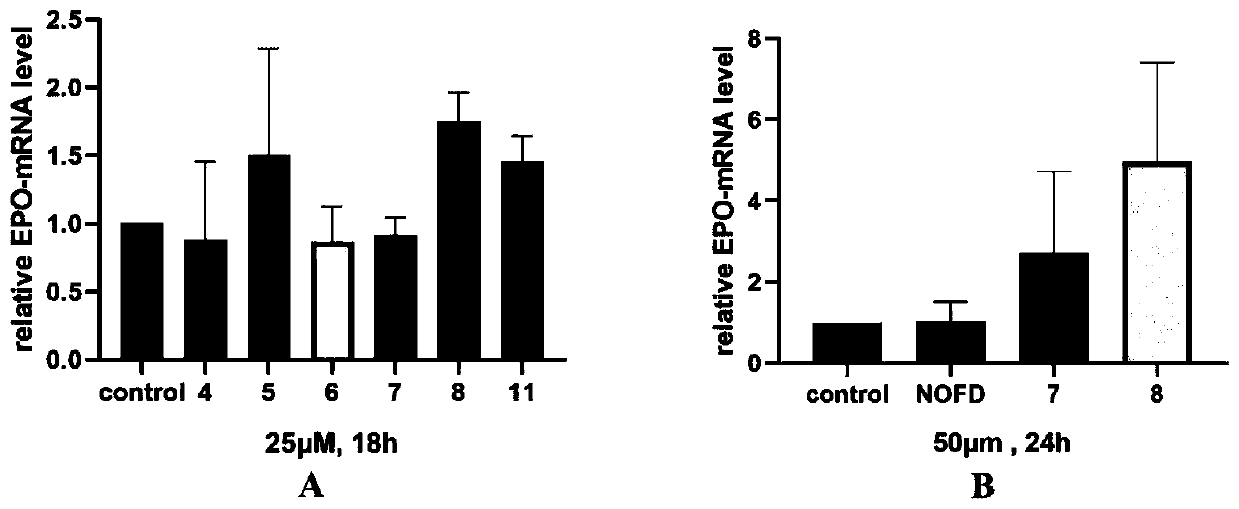
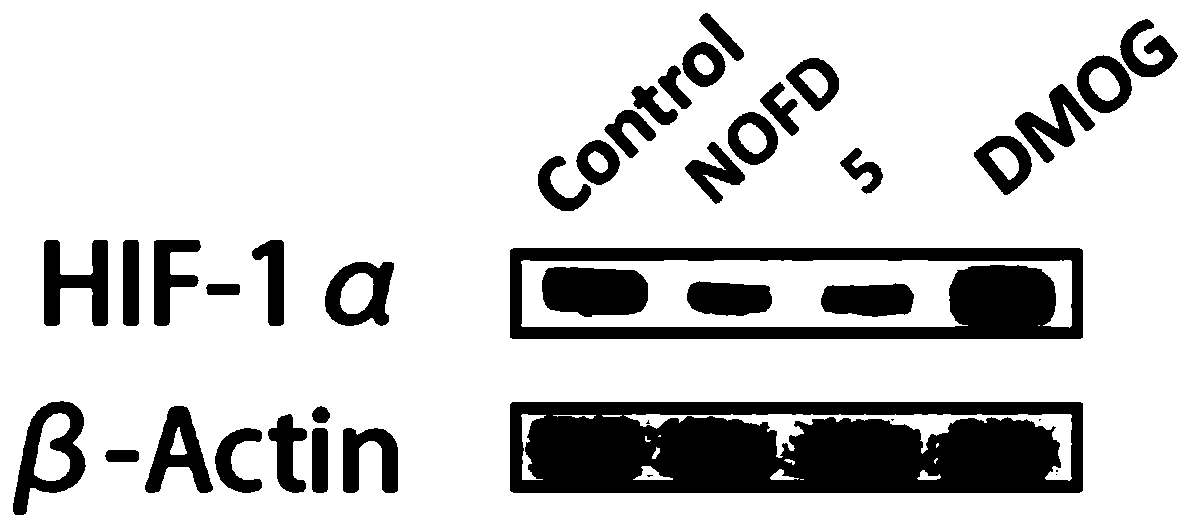
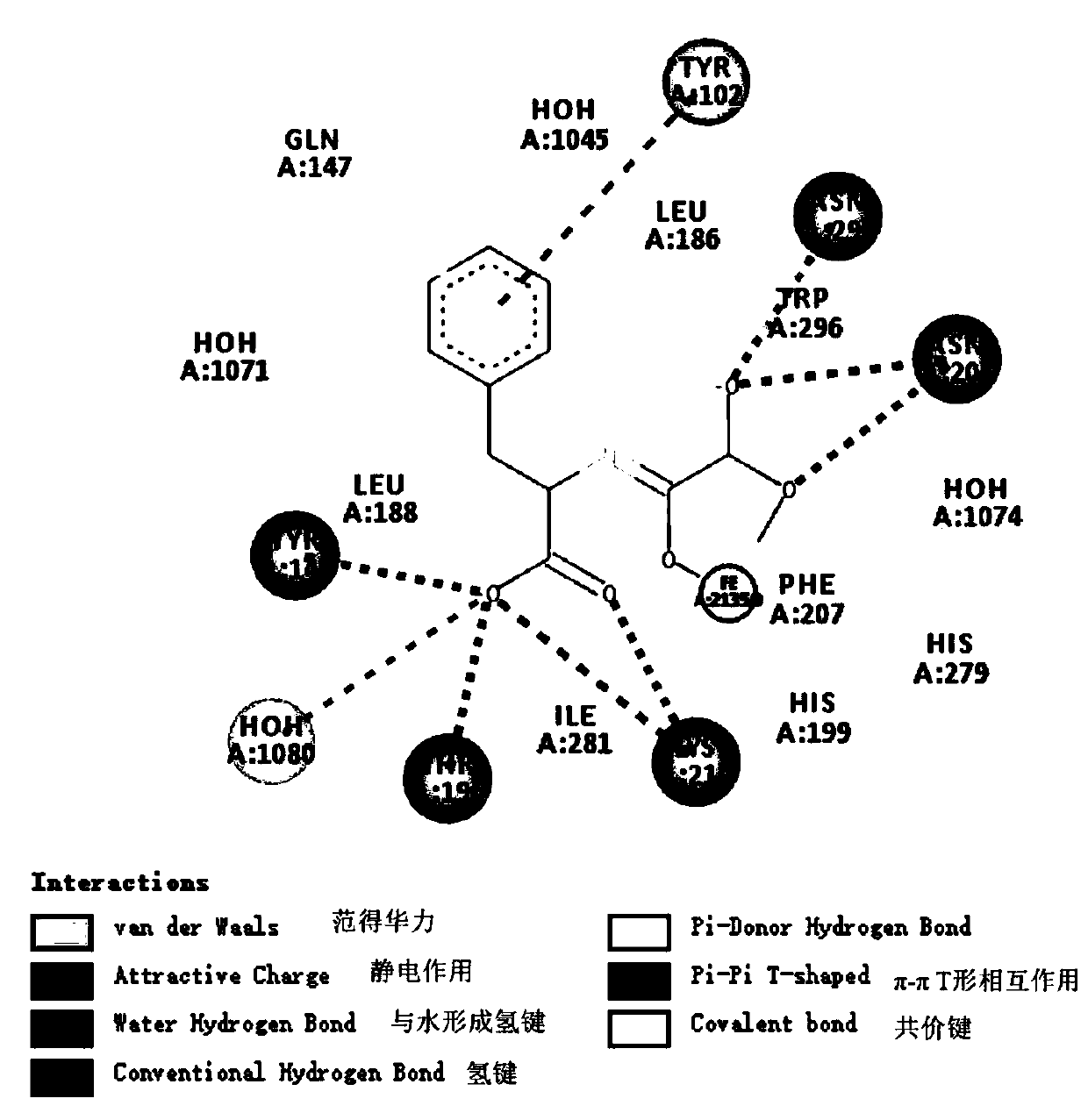
Image
Examples
Embodiment 1
[0039]
[0040] Methyl(6-bromopicolinoyl)phenylalaninate (Compound I-1): Add 20ml of anhydrous N,N-dimethyl phenyl formamide, followed by adding EDCI (2.81g, 14.7mmol, 1.5eq), HOBT (2.0g, 14.8mmol, 1.5eq), and then adding phenylalanine methyl ester hydrochloride (3.2g, 14.8mmol, 1.5eq) Finally, TEA (7.0g, 69mmol, 7.0eq) was added and reacted overnight (TLC detection end point). After the reaction, add water and stir, extract with ethyl acetate three times, wash with dilute hydrochloric acid three times, wash with saturated sodium bicarbonate solution three times, dry the organic phase with anhydrous sodium sulfate, and spin off ethyl acetate under reduced pressure to obtain a crude product. The crude product was purified by a flash silica gel column (eluent: petroleum ether: ethyl acetate = 10: 1 ~ petroleum ether: ethyl acetate = 1: 1) to obtain 2.9 g of a light yellow solid with a yield of 81%; 1H NMR (300MHz, DMSO) 68.89 (d, J=8.2Hz, 1H, -NH-), 8.75 (dd, J=2.2, 0.8 Hz, ...
Embodiment 2
[0042]
[0043] Methyl (5-bromopicolinoyl) phenylalaninate (compound I-2): under stirring at room temperature, 50 ml of anhydrous dichloromethane was added to 5-bromo-2-pyridinecarboxylic acid (2.81 g, 13.9 mmol, 1.5 eq), and then Add EDCI (2.67g, 13.9mmol, 1.5eq), HOBT (1.88g, 13.9mmol, 1.5eq) successively, then add phenylalanine methyl ester hydrochloride (2.0g, 9.28mmol, 1.0eq) and finally add TEA (6.7g, 65mmol, 7.0eq), reacted overnight (TLC detection end point). After the reaction, add water and stir, extract with ethyl acetate, wash with dilute hydrochloric acid and saturated sodium bicarbonate solution, dry the organic phase with anhydrous sodium sulfate, and spin off ethyl acetate under reduced pressure to obtain a crude product. The crude product was purified by a flash silica gel column (eluent: petroleum ether: ethyl acetate = 10: 1 ~ petroleum ether: ethyl acetate = 1: 1) to obtain 2.28 g of a light yellow solid with a yield of 68%; 1 H NMR (300MHz, DMSO) δ8.99...
Embodiment 3
[0045]
[0046] Methyl(5-bromopicolinoyl)tyrosinate (compound I-3):, under stirring at room temperature, 50ml of anhydrous dichloromethane was added to 5-bromo-2-pyridinecarboxylic acid (500mg, 2.48mmol, 1.0eq), followed by Add EDCI (721mg, 3.71mmol, 1.5eq), HOBT (502mg, 3.71mmol, 1.5eq), then add tyrosine methyl ester hydrochloride (860mg, 3.71mmol, 1.5eq) and finally add DIPEA (1.92g, 14.9mmol, 6.0eq), reacted overnight (TLC detection end point). After the reaction, add water and stir, extract with ethyl acetate, wash with dilute hydrochloric acid and saturated sodium bicarbonate solution, dry the organic phase with anhydrous sodium sulfate, and spin off ethyl acetate under reduced pressure to obtain a crude product. The crude product was purified by a flash silica gel column (eluent: petroleum ether: ethyl acetate = 10: 1 ~ petroleum ether: ethyl acetate = 1: 1) to obtain 293 mg of a light yellow solid with a yield of 32%; 1 H NMR (300 MHz, DMSO) 69.23 (s, 1H, -PhOH), 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com