Novel light stabilizer compound and preparation method and application thereof
A compound and composition technology, applied in the field of new light stabilizer compound and its preparation, can solve the problems of low rotational viscosity, insufficient service life, insufficient transmittance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
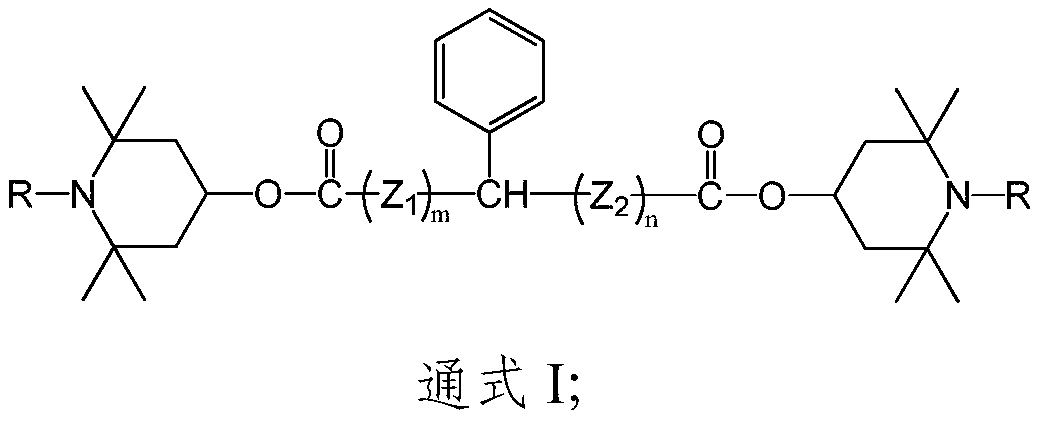
[0047] The present embodiment provides a kind of compound, and the structural formula of described compound is:
[0048]
[0049] This embodiment also provides the synthetic route for preparing compound BYLC-01 as follows:
[0050]
[0051] Specific steps are as follows:
[0052]Under nitrogen protection, add 31.5g 2,2,6,6-tetramethylpiperidin-4-ol, 18.0g 2-phenylmalonic acid, 3.0g tetradimethylaminopyridine, 350ml di Chloromethane, stirred for 0.5 hours, controlled temperature -10°C-5°C, added dropwise a solution consisting of 51.5g of dicyclohexylcarbodiimide and 150ml of dichloromethane, and reacted at room temperature for 6 hours. After conventional post-processing, chromatographic purification and n-hexane elution gave 40.5 g of a light pink solid (compound BYLC-01), LC: 99.7%, yield: 88.5%.
[0053] The obtained BYLC-01 was analyzed by LC-MS, and the m / z of the product was 458.1 (M+).
[0054] Elemental analysis: C: 70.70, H: 9.22, N: 6.12, O: 13.93.
Embodiment 2
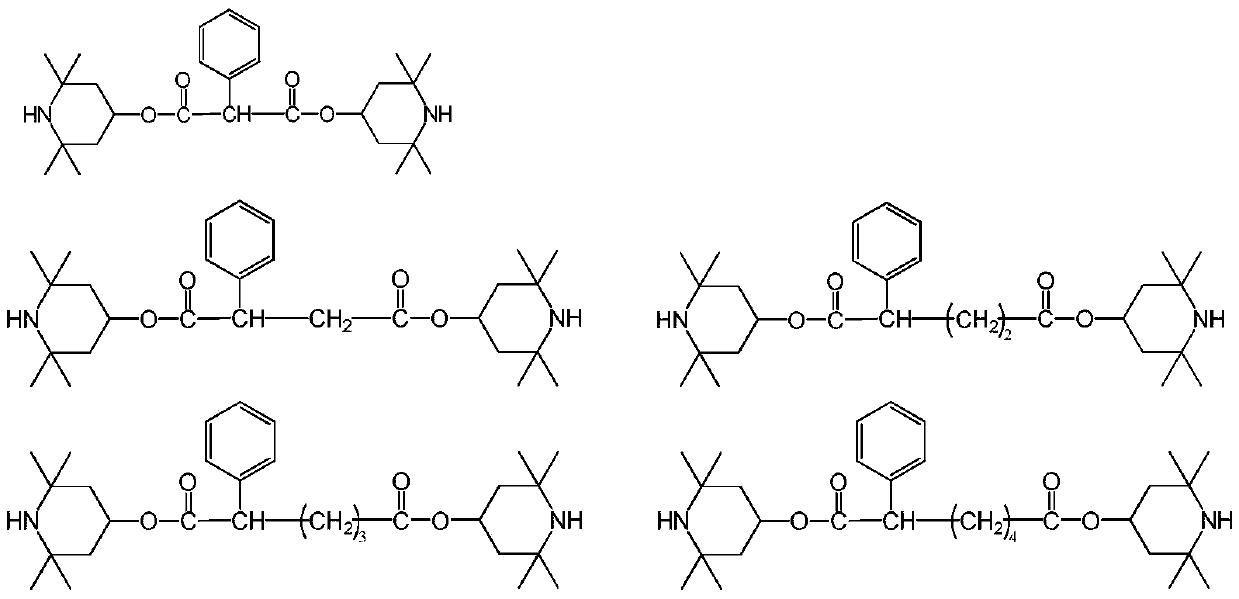
[0056] The present embodiment provides a kind of compound, and the structural formula of described compound is:
[0057]
[0058] This embodiment also provides the synthetic route for preparing compound BYLC-02 as follows:
[0059]
[0060] Specific steps are as follows:
[0061] Under nitrogen protection, add 47.2g 2,2,6,6-tetramethylpiperidin-4-ol, 31.2g 3-phenylglutaric acid, 3.6g tetradimethylaminopyridine, 350ml di Chloromethane, stirred for 0.5 hours, controlled temperature -5°C-5°C, added dropwise a solution consisting of 66.0 g of dicyclohexylcarbodiimide and 130 ml of dichloromethane, and reacted at room temperature for 8 hours. After conventional post-treatment, chromatographic purification and n-hexane elution gave 62.8 g of light pink solid (compound BYLC-02), LC: 99.8%, yield: 86.2%.
[0062] The obtained BYLC-02 was analyzed by LC-MS, and the m / z of the product was 486.1 (M+).
[0063] Elemental analysis: C: 71.55, H: 9.54, N: 5.75, O: 13.15.
Embodiment 3
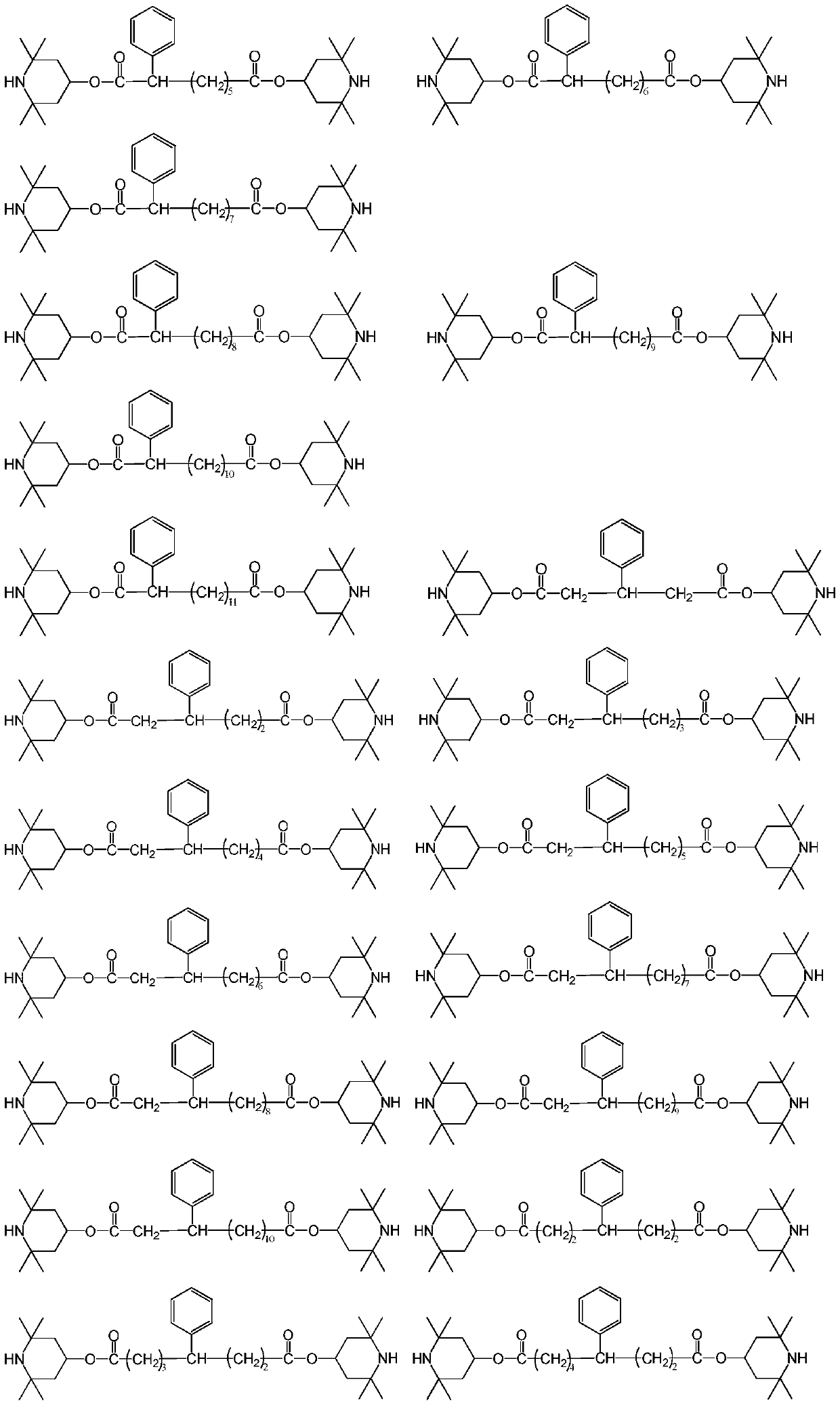
[0065] The present embodiment provides a kind of compound, and the structural formula of described compound is:
[0066]
[0067] This example also provides its preparation method: using 2,2,6,6-tetramethylpiperidin-4-ol and 2-phenylpimelic acid as raw materials, the reaction steps are the same as in Example 1, and the synthesis (compound BYLC-03) 40.0 g, LC: 99.8%, yield: 82.7%.
[0068] The obtained BYLC-03 was analyzed by LC-MS, and the m / z of the product was 514.1 (M+).
[0069] Elemental analysis: C: 72.30, H: 9.78, N: 5.43, O: 12.43.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com