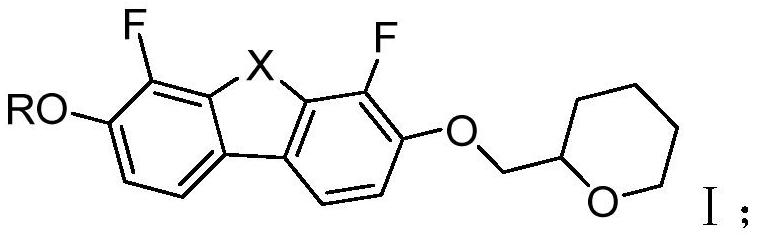
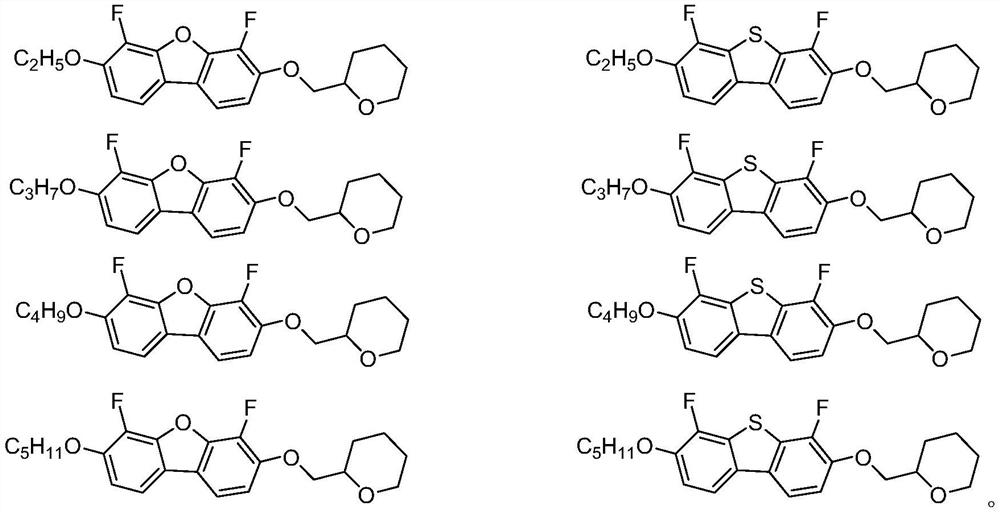
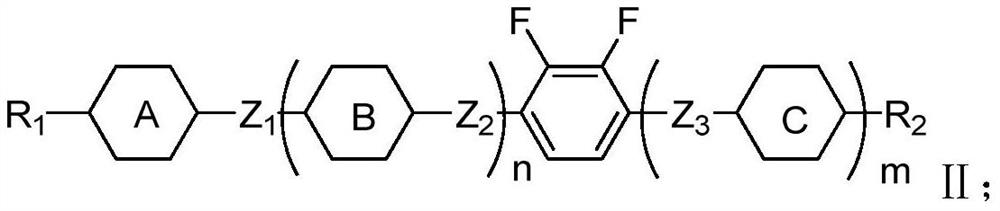
Negative dielectric anisotropy liquid crystal compound, composition and display element
A technology of liquid crystal compounds and liquid crystal compositions, applied in liquid crystal materials, organic chemistry, nonlinear optics, etc., can solve problems such as easy crystallization, poor compatibility, and application constraints, and achieve good liquid crystal phase stability and low rotation Effect of Viscosity, Wide Liquid Crystal Phase Range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Synthesis of 3-ethoxy-4,6-difluoro-7-((tetrahydro-2H-pyran-2-yl)methoxy)dibenzofuran (2OB(O)O1A)
[0079]
[0080] Step 1: Preparation of 2-(benzyloxy)-1-bromo-3-fluorobenzene (BrOBnG)
[0081]
[0082] Add 195.5 grams (1.023mol) of 2-fluoro-6-bromophenol, 282.5 grams (2.047mol) of potassium carbonate, 142.5 grams (1.13mol) of benzyl chloride and 1.0L DMF into a 2L three-necked flask, heat up to 75°C and stir the reaction 5 Hours, gas phase monitoring after no raw material remains, stop the reaction. Water was added to the reaction system, and extracted three times with n-heptane, the organic phases were combined, dried over anhydrous magnesium sulfate, filtered and spin-dried to obtain 280 g of liquid. Yield 97%, purity 98%.
[0083] Step 2: Preparation of 2'-(Benzyloxy)-4-ethoxy-2,3,3'-trifluoro-1,1'-biphenyl (2OYOBnG)
[0084]
[0085] In a 2L three-necked flask, 215.5 grams (1.07mol) of 2,3-difluoro-4-ethoxyphenylboronic acid, 272.5 grams (0.9...
Embodiment 2
[0107] Example 2: Synthesis of 3-butoxy-4,6-difluoro-7-((tetrahydro-2H-pyran-2-yl)methoxy)dibenzofuran (4OB(O)O1A)
[0108]
[0109] The method is similar to that of Example 1, except that in step 2, 2,3-difluoro-4-butoxyphenylboronic acid is used instead of 2,3-difluoro-4-ethoxyphenylboronic acid.
[0110] Structure Identification:
[0111] 1 H NMR (500MHz, CDCl 3 ):0.98~1.00(t,J=7.0Hz,3H),1.42~1.66(m,6H),1.72~1.75(m,1H),1.78~1.85(m,2H),1.89~1.93(m,1H ), 3.49~3.54(m,1H), 3.74~3.78(m,1H), 3.99~4.14(m,5H), 6.92~6.99(m,2H), 7.40~7.42(m,2H).
[0112] 13 C NMR (125MHz, CDCl 3 ): 13.8, 19.1, 23.0, 25.9, 28.2, 31.4, 68.5, 70.5, 74.3, 76.0, 111.3, 112.0, 114.4, 114.4, 120.2, 120.2, 138.2, 140.2, 144.7, 144.8, 146.3, 146.5.
[0113] MS m / z(RI,%):390.4(M + ,46), 236.2(100), 99.2(58), 292.2(17).
[0114] Thermal performance test: Cr 93.78I.
Embodiment 3
[0115] Example 3: Synthesis of 3-ethoxy-4,6-difluoro-7-((tetrahydro-2H-pyran-2-yl)methoxy)dibenzothiophene (2OB(S)O1A)
[0116]
[0117] Step 1: Preparation of 4'-ethoxy-2',3,3'-trifluoro-[1,1'-biphenyl]-2-yl triflate (2OYOSO2CF3G)
[0118]
[0119] Add 113 grams (421.6 mmol) of 2OYOHG, 72.4 grams (716.8 mmol) of triethylamine and 600 mL of dichloromethane into a three-necked flask, and after the system cools down to 5-10°C, start to add 166.5 grams (590.4 mmol) of trifluoromethanesulfonate dropwise After the addition of acid anhydride was completed, the reaction was continued to be stirred for 1 hour, and then the reaction was stopped, and water was slowly added dropwise to the system to quench it. After dichloromethane was extracted three times, the organic phases were combined, dried over anhydrous magnesium sulfate, filtered and spin-dried, and recrystallized from n-heptane to obtain 126 g of a white solid with a yield of 74.7% and a purity of 92%.
[0120] Step 2: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com