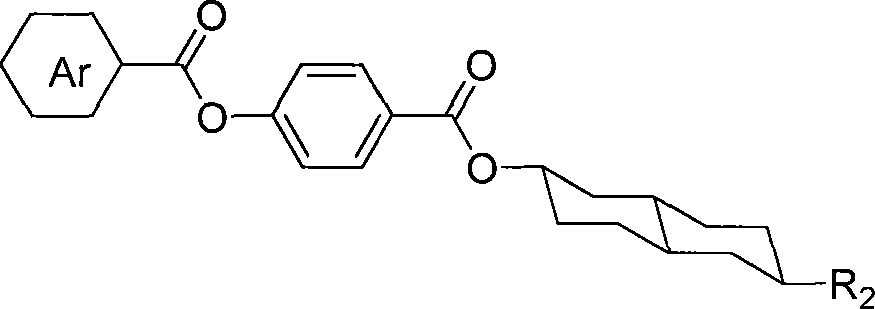
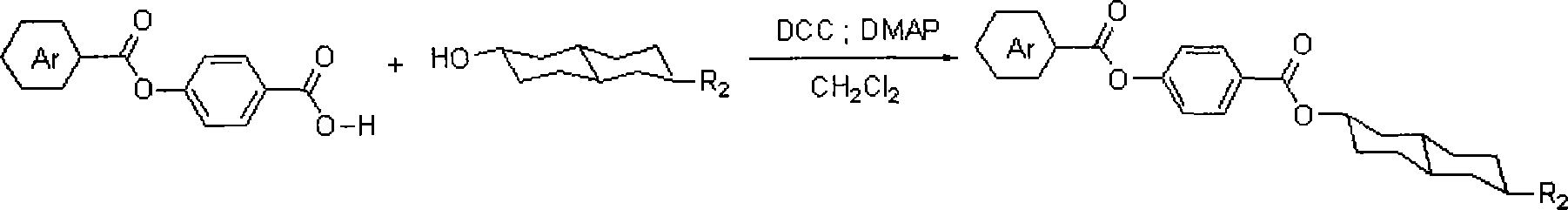Fluorine-containing trans-decalin esters compounds and synthesis method thereof
A technology of decahydronaphthyl esters and compounds, which is applied in the field of trans-decalin compounds and their synthesis, can solve the problems of narrow liquid crystal phase intervals and cannot meet the requirements, and achieve wide liquid crystal phase intervals, easy separation, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Preparation of 4-(4'-fluorobenzoyloxy)benzoic acid (6-n-pentyl) trans-decalin-2-ester
[0017] English name: 4-((6’-pentyl-decahydronaphthalen-2-yloxy)carbonyl)phenyl4-fluorobenzoate
[0018] Structural formula:
[0019]
[0020] Subnumerary: 466.58
[0021] Appearance: white solid
[0022] Melting point: 113.69°C
[0023] Clearing point: 213.07℃
[0024] Infrared spectrum (using Perkin-Elmer983G infrared spectrometer, KBr tablet)
[0025] υ max (cm -1 ): 2918, 2850, 1730, 1709, 1604, 1510, 1463, 1266, 1205, 1163, 1116, 1064, 1012, 885, 846, 762
[0026] NMR spectrum 1 H spectrum (500 MHz, deuterated chloroform) δ / ppm: 8.22 (m, 2H, ArH), 8.10 (m, 2H, Ar H ), 7.28 (m, 2H, Ar H ), 7.19 (m, 2H, Ar H ), 4.97 (m, 1H, OC H ), 0.63-2.16(m, 27H)
[0027] NMR spectrum 13 C spectrum (126 MHz, deuterated chloroform) δ / ppm: 167.45, 165.41, 163.39, 154.39, 133.07, 133.00, 131.33, 128.83, 125.53, 125.51, 121.72, 116.14, 115.97, 74.28, 42.19, 40.9. , 37.49...
example 2
[0029] Example two: with dry dichloromethane as solvent, add 6-n-pentyl-2-hydroxyl-trans decahydronaphthalene (3.5g, 15.6mmol), 4-(4-fluoro Benzoyloxy)benzoic acid (4.5g, 1.72mmol) DCC (3.5g, 17.2mmol) and DMAP (2.1g, 17.2mmol), after stirring at room temperature for 10 hours, stop the reaction, filter, and remove the solvent under reduced pressure Column chromatography (petroleum ether: ethyl acetate = 12:1). The resulting product was recrystallized with ethanol to obtain 5.4 grams of a white solid to obtain the compound 4-(4'-fluorobenzoyloxy)benzoic acid (6-n-pentyl)trans-decalin-2-ester with a yield of 74% .
Embodiment 3
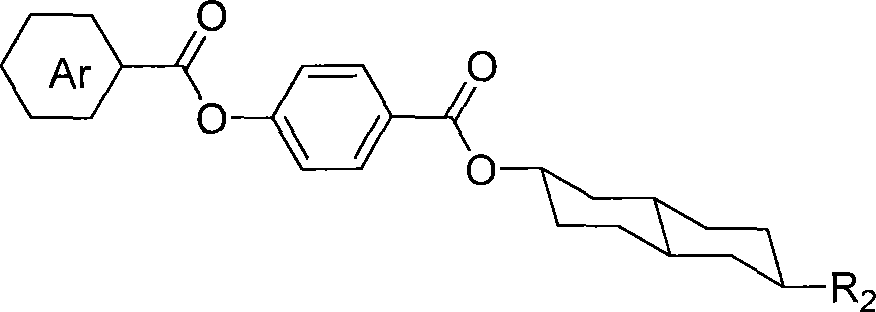
[0030] Example 3: Preparation of 4-(4-n-heptyloxy-benzoyloxy)benzoic acid (6-n-pentyl)trans-decalin-2-ester. The structural formula is:
[0031]
[0032] Molecule: 534.73, appearance: white solid, melting point: 82.9°C, clearing point: 199.5°C
[0033] Spectral data:
[0034] Infrared spectrum (using Perkin-Elmer983G infrared spectrometer, KBr tablet)
[0035] υ max (cm -1 ): 2918, 2850, 1730, 1709, 1604, 1510, 1463, 1266, 1205, 1163, 1116, 1064, 1012, 885, 846, 762
[0036] NMR spectrum 1 H spectrum (500 MHz, deuterated chloroform) δ / ppm: 8.13 (d, 2H, ArH), 8.10 (d, 2H, Ar H ), 7.27 (d, 2H, Ar H ), 6.97(d, 2H, Ar H ), 4.96 (m, 1H, OC H ), 4.04(t, 2H, OC H 2 ), 0.63-2.16(m, 39H)
[0037] NMR spectrum 13C spectrum (126 MHz, deuterated chloroform) δ / ppm: 165.53, 164.58, 163.89, 154.77, 132.52, 131.24, 128.51, 121.85, 121.24, 114.52, 74.21, 68.51, 42.13, 41.22, 450.09, 37.81, 37.11, 3 , 33.68, 33.09, 32.35, 32.09, 31.91, 31.86, 29.24, 29.18, 26.76, 26.09, 22.86, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| isotropization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com