Light stabilizer and preparation method and application thereof
A light stabilizer, esterification technology, applied in chemical instruments and methods, organic chemistry, liquid crystal materials, etc., can solve the problems of increased conductivity, insufficient stability of liquid crystal media, damage to stable electrical properties, etc., and achieve high specific resistance. value, good liquid crystal mutual solubility, good effect of rotational viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
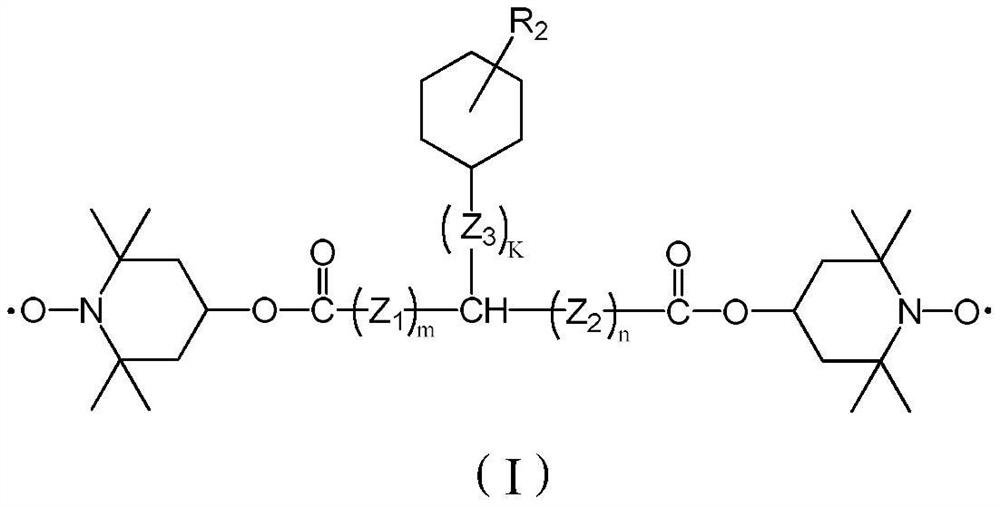
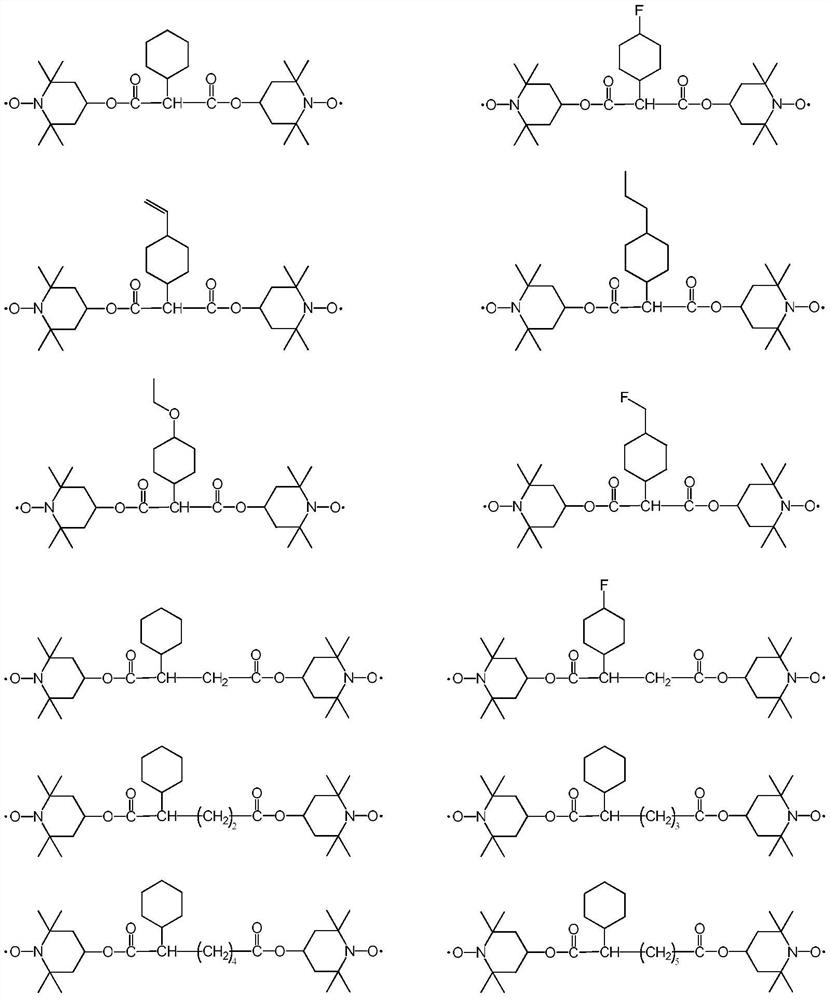
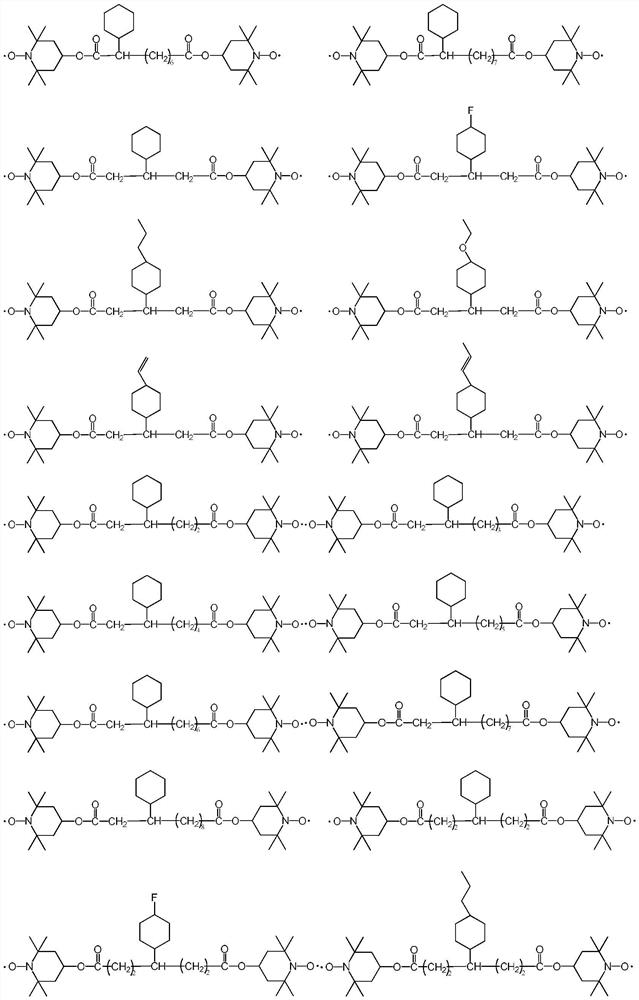
[0049] The present embodiment provides a kind of compound, and the structural formula of described compound is:
[0050]
[0051] This embodiment also provides the synthetic route for preparing compound BYLC-01 as follows:
[0052]
[0053] Specific steps are as follows:
[0054]Under nitrogen protection, add 51.6g 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl, 27.5g 2-cyclohexylmalonic acid, 3.7g 4-dimethylamine to the reaction flask basepyridine, 400ml of dichloromethane, stirred for 0.5 hours, controlled temperature -10°C-5°C, added dropwise a solution consisting of 66.0g of dicyclohexylcarbodiimide and 150ml of dichloromethane, and reacted at room temperature for 6 hours. After conventional post-treatment, chromatographic purification and n-hexane elution gave 61.3 g of light pink solid (compound BYLC-01), LC: 99.7%, yield: 82.8%.
[0055] The obtained BYLC-01 was analyzed by LC-MS, and the m / z of the product was 494.1 (M+).
[0056] Elemental analysis: C: 65.55, ...
Embodiment 2
[0058] The present embodiment provides a kind of compound, and the structural formula of described compound is:
[0059]
[0060] This embodiment also provides the synthetic route for preparing compound BYLC-02 as follows:
[0061]
[0062] Specific steps are as follows:
[0063] Under nitrogen protection, add 43.0g 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl, 26.7g 3-cyclohexylglutaric acid, 1.8g 4-dimethylamine to the reaction flask Basepyridine, 500ml of dichloromethane, stirred for 0.5 hours, controlled temperature -5°C-5°C, added dropwise a solution consisting of 31.5g of dicyclohexylcarbodiimide and 80ml of dichloromethane, and reacted at room temperature for 8 hours. After conventional post-treatment, chromatographic purification and n-hexane elution gave 57.6 g of a light pink solid (compound BYLC-02), LC: 99.7%, yield: 88.3%.
[0064] The obtained BYLC-02 was analyzed by LC-MS, and the m / z of the product was 522.1 (M+).
[0065] Elemental analysis: C: 66.63...
Embodiment 3
[0067] The present embodiment provides a kind of compound, and the structural formula of described compound is:
[0068]
[0069] This embodiment also provides its preparation method: using 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl and 4-cyclohexylpimelic acid as raw materials, the reaction steps are the same as those in Example 1. Example 2, synthesis (compound BYLC-03) 55.4g, LC: 99.6%, yield: 82.5%.
[0070] The obtained BYLC-03 was analyzed by LC-MS, and the m / z of the product was 550.1 (M+).
[0071] Elemental analysis: C: 67.60, H: 9.88, N: 5.09, O: 17.43.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com