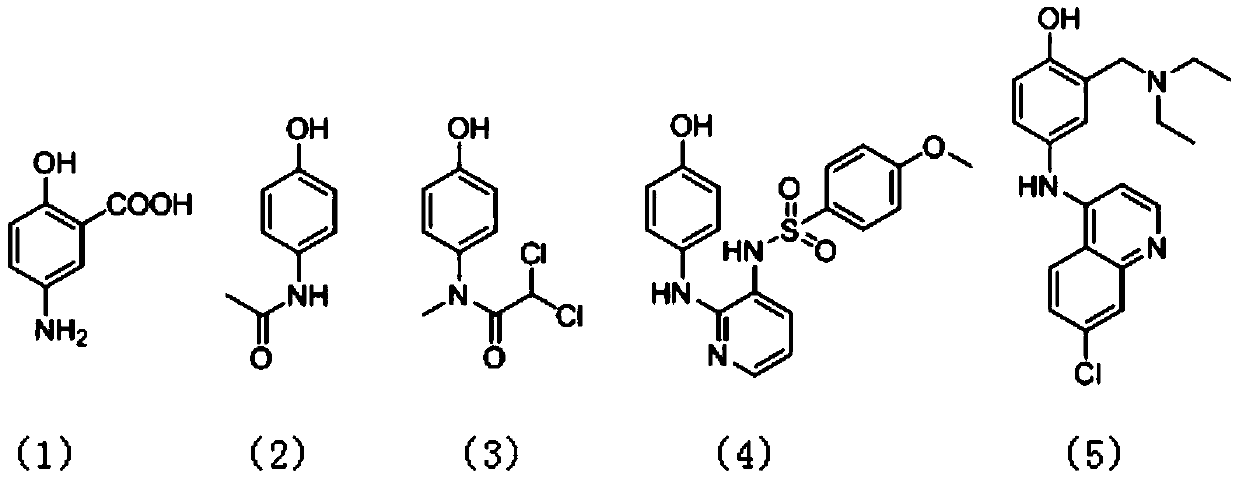
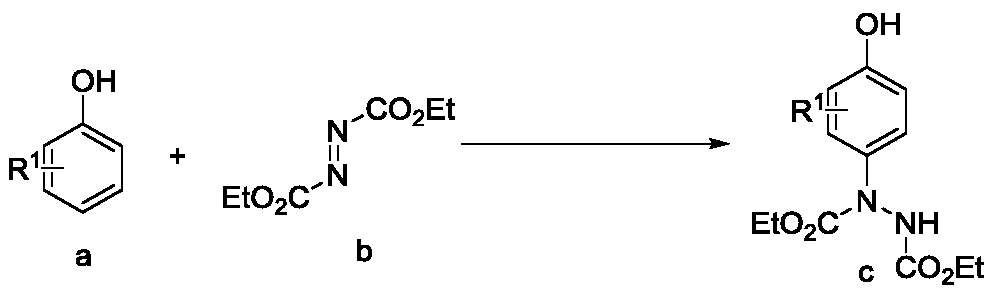
Preparation method of p-amino substituted phenol compound
A technology for phenolic compounds and p-amino groups, applied in the direction of organic chemistry, can solve few problems and achieve the effects of short reaction time, good regioselectivity, and simple and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
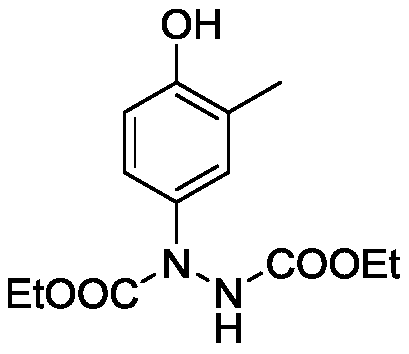
Embodiment 1
[0022] Preparation of Diethyl 1-(4-Hydroxy-3-methylphenyl)hydrazine-1,2-dicarboxylate
[0023]
[0024] Add catalyst 3mol% Ag sequentially 2 O, 0.2mmol o-cresol, 0.4mmol diethyl azodicarboxylate, 2mL water and a No. 5 magnet. The reactor was placed in an ice bath to react for 0.5 hours. The reaction solution was poured into a separatory funnel, 15 mL of water was added, extracted 3 times with 10 mL of ethyl acetate, the resulting organic phases were combined, spin-dried by a rotary evaporator, and the crude product was separated and purified by column chromatography to obtain 1-(4-hydroxyl -32.1 mg of diethyl 3-methylphenyl)hydrazine-1,2-dicarboxylate, as a white solid, with a yield of 57%.
[0025] Its structure was confirmed by H NMR and C NMR 1 H NMR (400MHz, CDCl 3 ): δ7.19(s,1H),7.12(s,1H),7.02(d,J=7.80Hz,1H),6.62(d,J=8.56Hz,1H),6.21(s,1H),4.25 –4.16(m,4H),2.17(s,3H),1.28–1.22(m,6H); 13 C NMR (100MHz, CDCl 3 ): δ156.6, 155.9, 153.5, 134.0, 128.3, 124.8, 115.1, 6...
Embodiment 2
[0027] Preparation of Diethyl 1-(4-Hydroxy-3-methoxyphenyl)hydrazine-1,2-dicarboxylate
[0028]
[0029] Add catalyst 3mol% Ag sequentially 2 O, 0.2mmol 2-fluorophenol, 0.4mmol diethyl azodicarboxylate, 2mL water and a No. 5 magnet. The reactor was placed in an ice bath to react for 0.5 hours. The reaction solution was poured into a separatory funnel, 15 mL of water was added, extracted 3 times with 10 mL of ethyl acetate, the resulting organic phases were combined, spin-dried by a rotary evaporator, and the crude product was separated and purified by column chromatography to obtain 1-(4-hydroxyl -44.7 mg of diethyl 3-methoxyphenyl)hydrazine-1,2-dicarboxylate, a white solid, with a yield of 75%.
[0030] Its structure was confirmed by H NMR and C NMR 1 H NMR (400MHz, CDCl 3 ):δ7.24(s,1H),7.01(s,1H),6.88–6.84(m,1H),6.83(d,J=8.44Hz,1H),5.83(s,1H),4.24–4.18( m,4H),3.84(s,3H),1.28–1.22(m,6H); 13 C NMR (100MHz, CDCl 3 ): δ156.5, 155.5, 146.3, 144.8, 134.3, 114.1, 63.0, 62...
Embodiment 3
[0032] Preparation of Diethyl 1-(3-fluoro-4-hydroxyphenyl)hydrazine-1,2-dicarboxylate
[0033]
[0034] Add catalyst 3mol% Ag sequentially 2 O, 0.2mmol 2-fluorophenol, 0.4mmol diethyl azodicarboxylate, 2mL water and a No. 5 magnet. The reactor was placed in an ice bath to react for 0.5 hours. The reaction solution was poured into a separatory funnel, 15 mL of water was added, extracted 3 times with 10 mL of ethyl acetate, the resulting organic phases were combined, spin-dried by a rotary evaporator, and the crude product was separated and purified by column chromatography to obtain 1-(3-fluoro -45.2 mg of diethyl 4-hydroxyphenyl)hydrazine-1,2-dicarboxylate, a white solid, with a yield of 79%.
[0035] Its structure was confirmed by H NMR and C NMR 1 H NMR (400MHz, CDCl 3 ):δ7.24(s,1H),7.17(d,J=11.64Hz,1H),7.03(d,J=8.88Hz,1H),6.89–6.83(m,1H),6.31(s,1H) ,4.26–4.19(m,4H),1.29–1.24(m,6H); 13 C NMR (100MHz, CDCl 3 ): δ156.6, 155.3, 150.4 (d, J = 238.12Hz), 142.8, 134.3, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com