Pharmaceutical composition for treating constipation containing l-arabinose and its application method
A kind of arabinose, a technology for treating constipation, applied in the field of biomedicine, can solve problems such as unsuitable for long-term use, drug dependence, weak effect, etc., and achieve the effect of increasing intestinal water content, promoting peristalsis, and preventing rapid loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
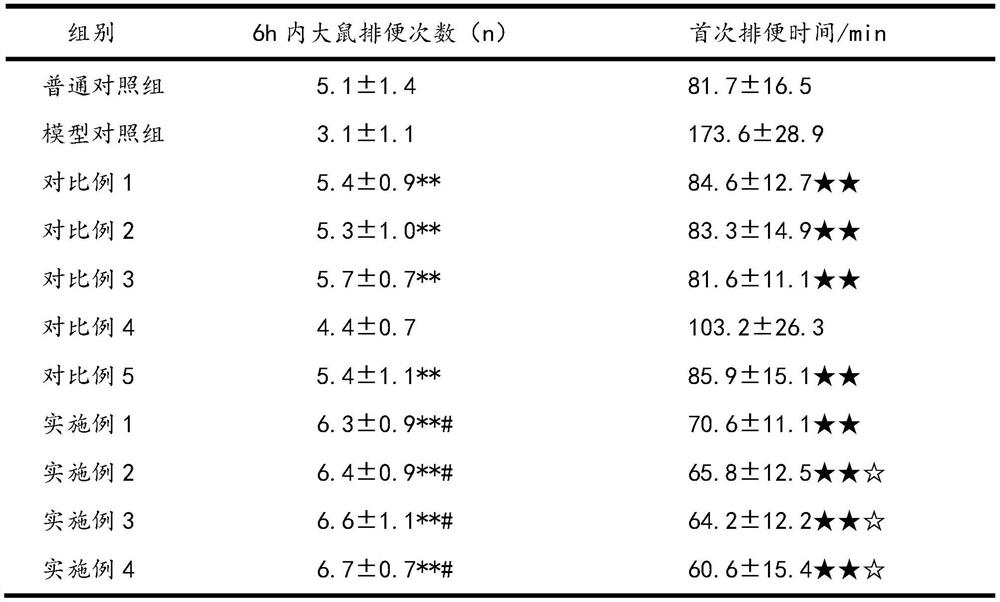
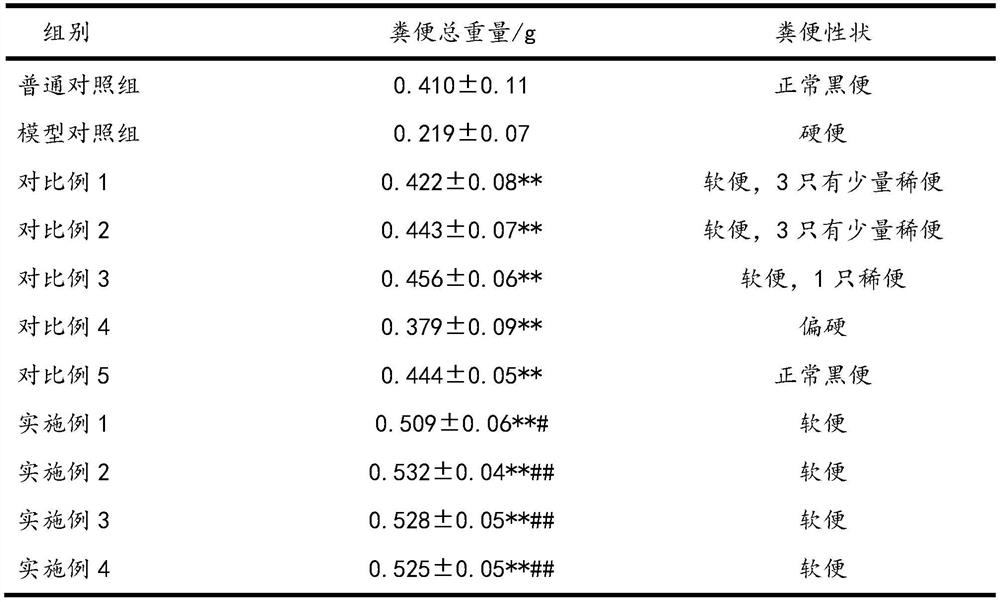
[0026] The impact experiment of pharmaceutical composition on constipation mouse defecation is as follows:
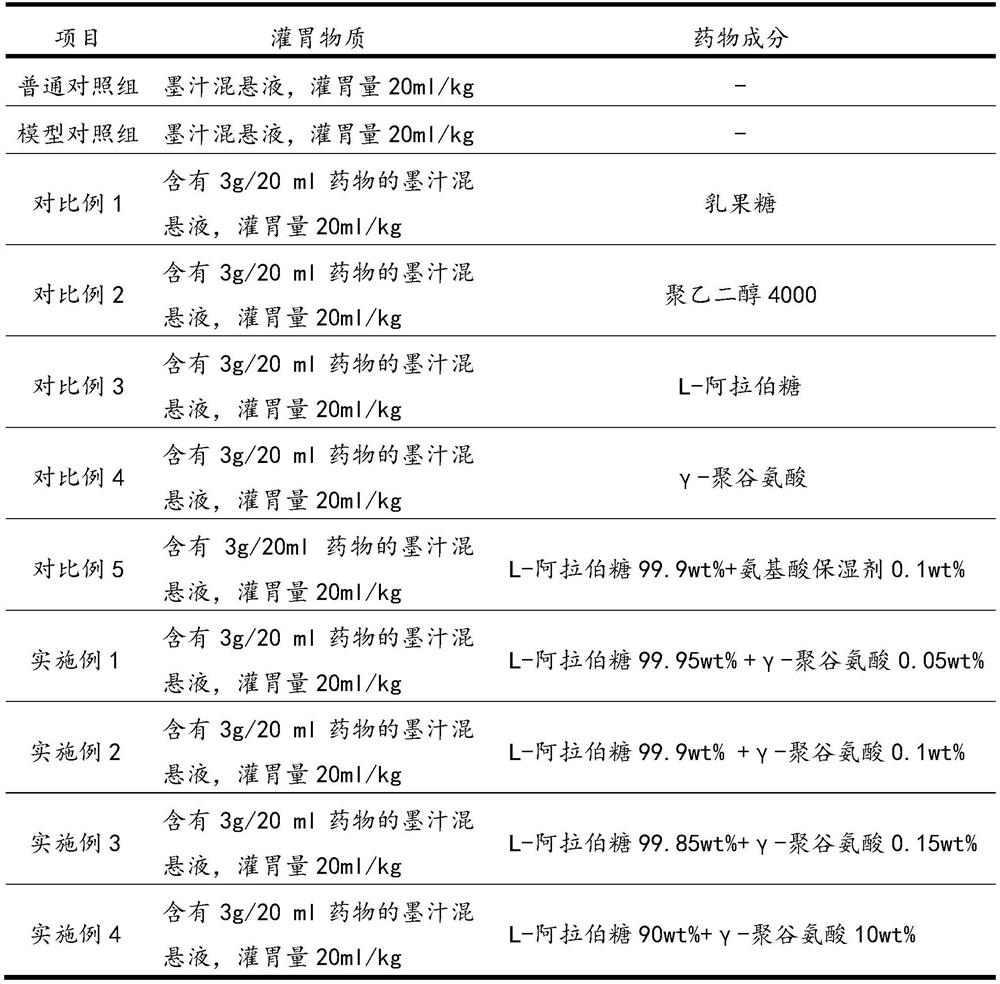
[0027] Take 110 clean-grade mice (experimental mice were purchased from the Experimental Animal Center of Xiamen University, license number SYXK (Fujian) 2018-0010), weighing 20±2g, male and female, and randomly divided into 11 groups, 10 in each group. The specific groups are Example 1, Example 2, Example 3, Example 4, normal control group, model control group, comparative example 1, comparative example 2, comparative example 3, comparative example 4, and comparative example 5.
[0028] Among them, except for the normal control group, the other 10 groups used the compound diphenoxylate to establish the constipation mouse model, and the dosage of the compound diphenoxylate was 10 mg / kg.
[0029] 12 hours before the start of the test, food and water were not allowed. The experiment was administered according to Table 1. The timing was started after the gavage. Each mouse...
experiment example 2
[0050] The pharmaceutical composition of the present invention is made into 3g / bag powder (99.9wt% of L-arabinose and 0.1wt% of gamma-polyglutamic acid) for trial use by people with constipation. A total of 42 cases of trial people are used for voluntary trial of constipation. patient.
[0051] Table 4. Basic information of trial patients (age range 27-65 years old)
[0052]
[0053] The classification criteria for the degree of constipation in patients with constipation are as follows:
[0054] Mild constipation: defecate every other day, the stool is dry; defecation is laborious;
[0055] Moderate constipation: generally have a bowel movement every 2-3 days, the stool is dry, and occasionally blood is present during defecation;
[0056] Severe constipation: usually only once a week for 4-5 days or even a week, and the defecation volume is small and the defecation process is painful, often need to rely on lubricants such as Kaisailu to complete defecation;
[0057] expe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com