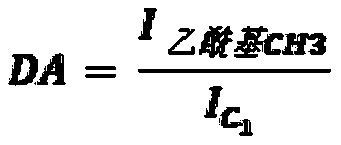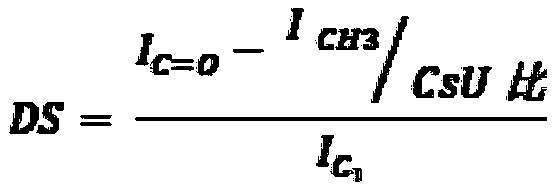Anionically charged chitosan
A technology of chitosan and polysaccharide, applied in the field of carboxyalkyl chitosan, can solve problems such as difficult implementation on an industrial scale, difficulty in implementation, and expensive energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0129] The invention also relates to a preparation method for preparing carboxyalkyl chitosan.
[0130] According to a variant, the production process for the preparation of carboxyalkyl chitosan according to the invention comprises the preparation of chitosan of fungal origin, reacetylation of the chitosan and carboxyalkylation of the reacetylated chitosan. Accordingly, the present invention relates to a reacetylated chitosan or reacetylated carboxyalkyl chitosan.
[0131] Thus, according to one embodiment, chitosan may be dissolved in an aqueous medium, preferably in a slightly acidic (eg pH 6) aqueous medium. Acetic anhydride can be added to the chitosan solution one or more times. An alkaline agent such as sodium hydroxide and / or urea is then added. An alkylating agent, such as sodium monochloroacetate (ie sodium chloroacetate) or chloroacetic acid, is then added. Subsequently, the substituted chitosan is purified, recovered and dried.
[0132] According to a variant, ...
Embodiment 1
[0237] Example 1 N-succinyl-chitosan
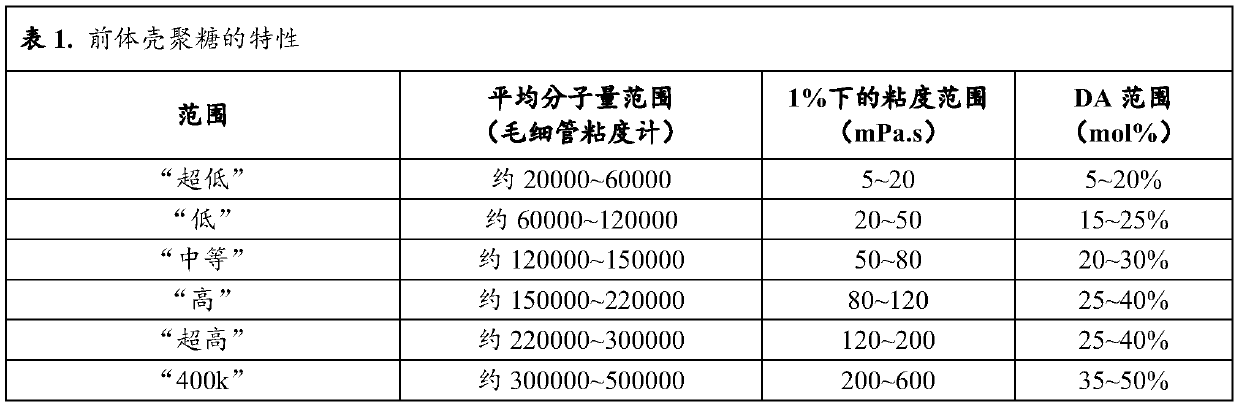
[0238] N with different molecular weights and molecular structures (degree of acetylation (DA) and degree of succinyl substitution (DS)) were prepared and characterized according to the general method described in patent application WO 2016 / 016463 or WO 2016 / 016464 (patent EP 3016663). - Succinochitosan. Except for polymer CSS-1 (Table 1a), the first acetylation step was performed by adding acetic anhydride to increase the degree of acetylation.
[0239] Formulations were prepared with these N-succinyl chitosan (CSS) at a concentration of 2% at physiological pH and osmolarity and packaged in glass syringes. Syringes were sterilized by autoclaving with a standard cycle (15 minutes at 121°C). According to the EP2.9.20 method, the formulations are checked visually to confirm that they are free of any insoluble matter or turbidity over a broad pH range around physiological pH (pH 6.5 to 7.5). Check that the level of bacterial endotoxin in ...
Embodiment 2
[0247] Example 2 - carboxymethyl chitosan of animal origin
[0248] Two carboxymethyl chitosans of animal origin (CC-1 and CC-2 in Table 2a) are available for implantable or injectable pharmaceutical or medical use.
[0249] Two other carboxymethyl chitosans were tested:
[0250] A CC (CC-3) obtained by carboxymethylation of chitin of animal origin,
[0251] A CC (CC-4) obtained by carboxymethylation of chitosan of animal origin prepared by deacetylation of chitin of animal origin.
[0252] Carboxymethyl chitosan (CC) was characterized by carbon 13 NMR to determine it and the values of DA (degree of acetylation) and DS (degree of substitution of carboxymethyl groups) were measured with an error range estimated to be about ±10 % (Table 2a). The electrostatic charge of the polymer was characterized by measuring the zeta potential at a concentration of 0.05% (m / m) in the range from pH 8 to pH 4, and the zeta potential value was recorded at a pH of 7.5 (Table 2a). It was obs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com