Recombinant human PD-1 antibody and application thereof
A PD-1, antibody technology, applied in recombinant DNA technology, applications, antibodies, etc., can solve the problem of different antigenic epitopes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
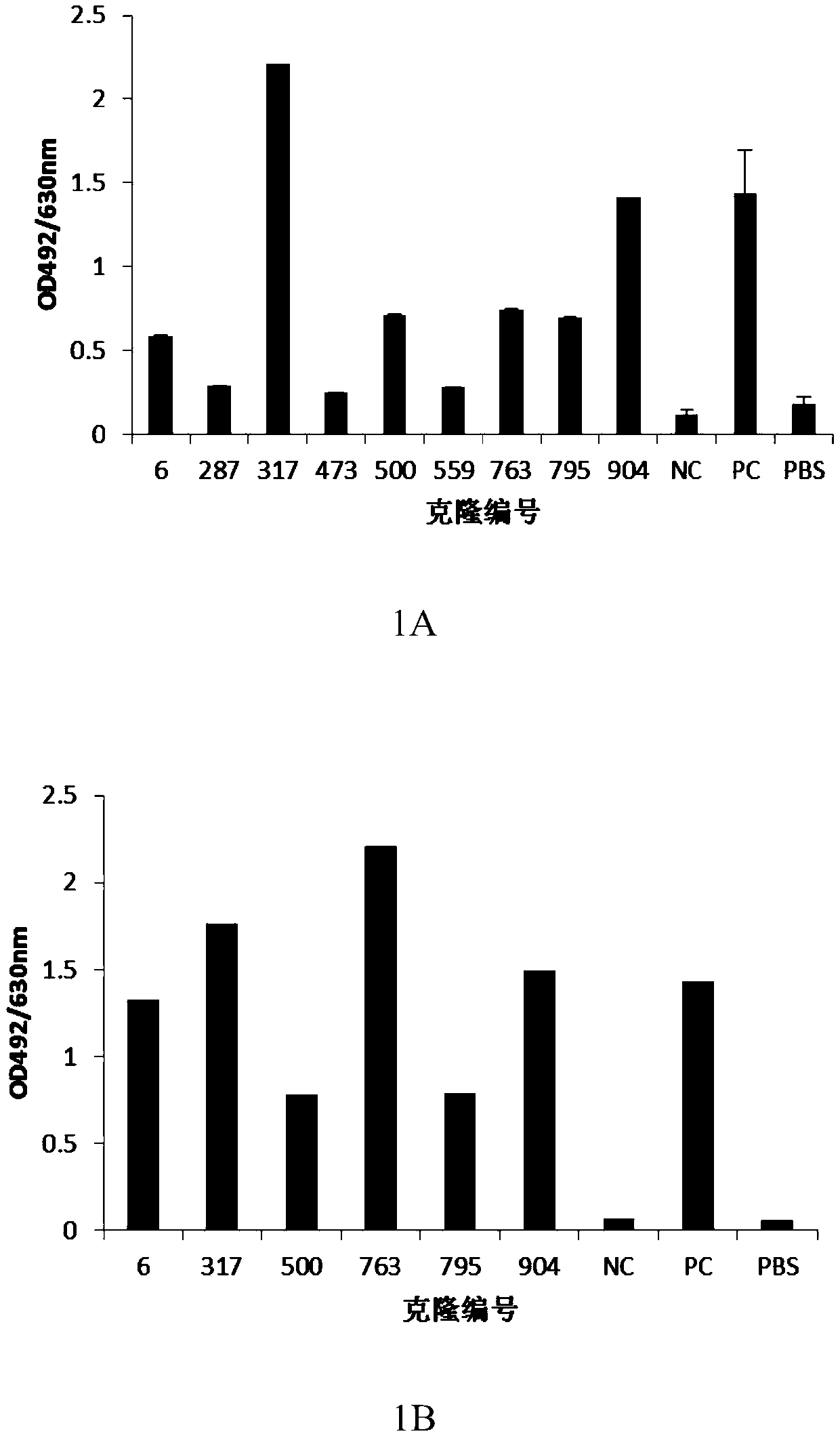
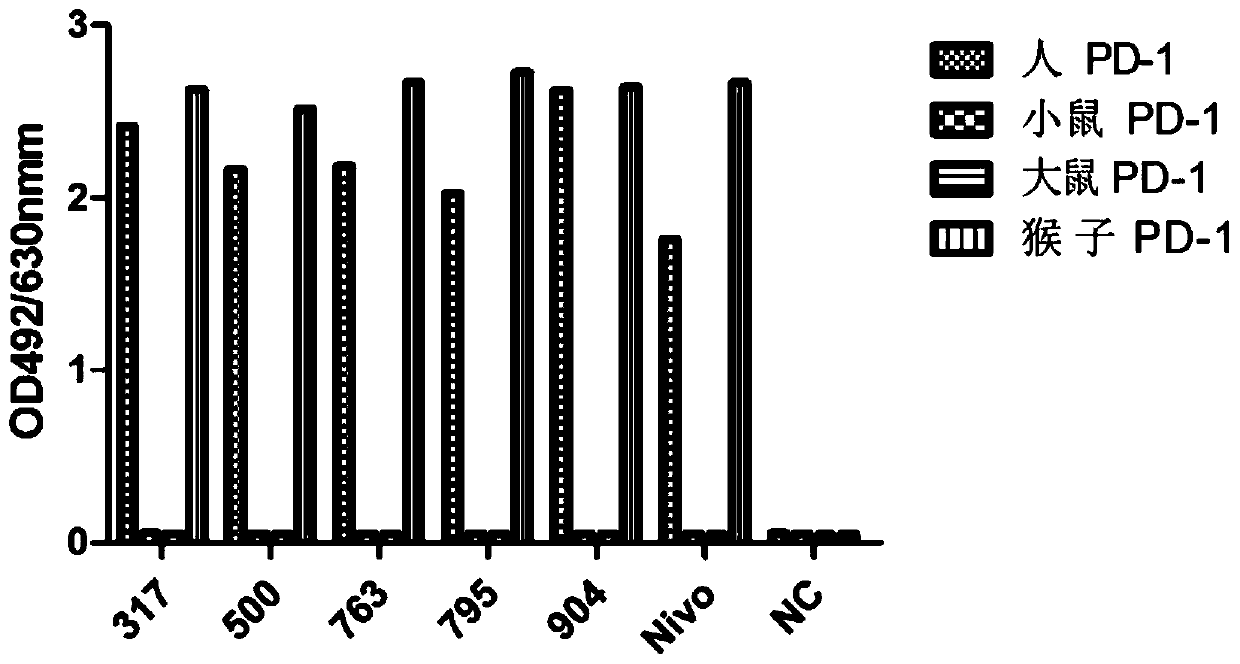
[0097] Example 1: Screening, identification and antibody sequence determination of anti-human PD-1 antibody hybridoma cell lines
[0098] Immunization: Freund's adjuvant and water-soluble adjuvant were used for immunization of mice. Freund's adjuvant was used for intraperitoneal injection twice on day 0 and day 14, and water-soluble adjuvant was administered on day 0 and day 14. In 21 days, 8-10 week-old Balb / c mice were given two intramuscular immunization injections, and the immune antigen was: human PD-1 / mFc recombinant protein (serial number: NP_005009.2, 21aa-167aa, Lot: 2016.3. 16), recombinantly expressed in HEK293 cells by Beijing Kenuo Xincheng Technology Co., Ltd. The dose for the first immunization was 50 μg, and the dose for the second immunization was 25 μg. The mouse serum was taken before immunization as a negative control for detection, Freund's adjuvant was used on the 28th day after the initial immunization, and water-soluble adjuvant was used to collect b...
Embodiment 2
[0109] Example 2: Preparation of anti-human PD-1 chimeric antibody
[0110] The monoclonal antibody light chain variable region and heavy chain variable region genes obtained by cloning were introduced into restriction sites by PCR with the following primers (Table 3), and cloned into human-kappa light chain constant region and human IgG4 heavy chain constant region respectively. In the eukaryotic expression vector upstream of the region coding gene, the human-mouse chimeric light chain (pKN019-317L) and human-mouse chimeric heavy chain (pKN034-317H) expression plasmids were obtained, which were transformed into Escherichia coli for amplification, and a large amount of Plasmids containing the light and heavy chains of the human-mouse chimeric antibody were mixed with 293fectin and co-transfected into HEK293 cells. Cells were transfected for 5-6 days, and the culture supernatant was taken, and the expression supernatant was purified by ProA affinity chromatography column, and...
Embodiment 3
[0116] Example 3: Humanization of anti-human PD-1 monoclonal antibody and construction of stable cell lines
[0117]Firstly, the heavy chain sequence of the murine antibody was comprehensively analyzed to determine the complementarity determinant (CDR) region where the antibody binds to the antigen and the framework region (framework) that supports the conservative three-dimensional conformation of the antibody. Then, according to the homology comparison results, the most similar human antibody template was found in the human antibody germline library (http: / / www2.mrc-lmb.cam.ac.uk / vbase / alignments2.php#VHEX), and VH3( 3-21) as the basic template, combined with the results of the full sequence blast, considering the frequency of amino acid occurrences (A49) of the rearranged antibody in a specific FR region, and performing CDR transplantation, considering that the FR3 region (S98) is close to the CDR3 region, no replacement According to the CDR3 sequence (Pdssgvay), JH4 (wgq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com