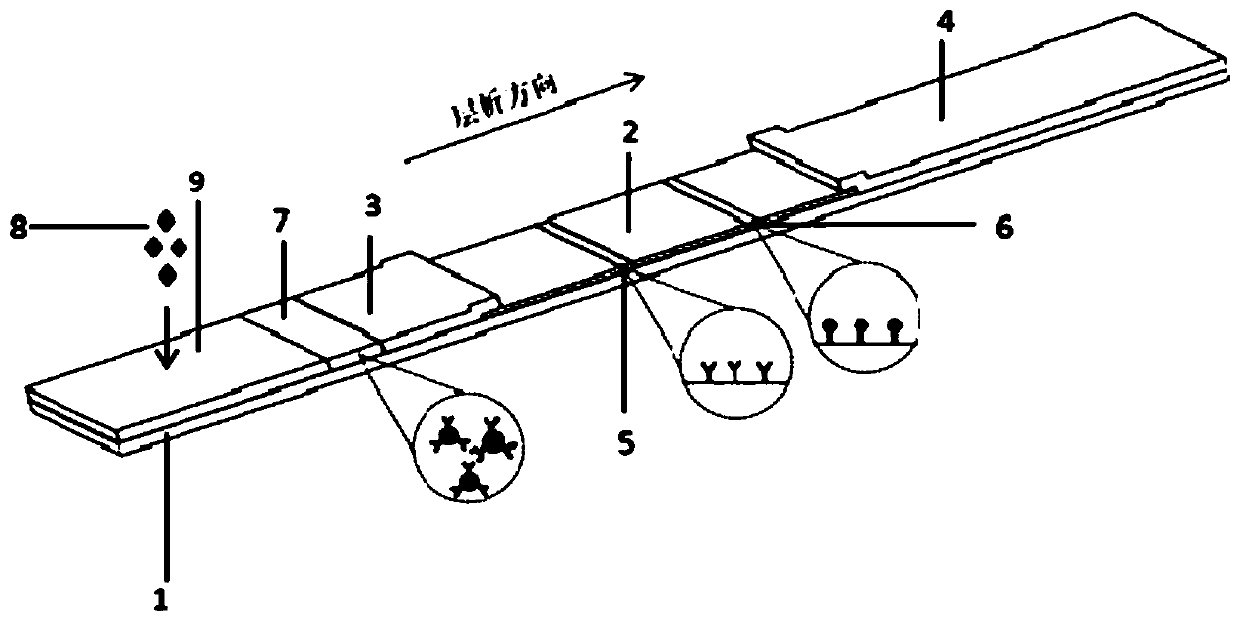
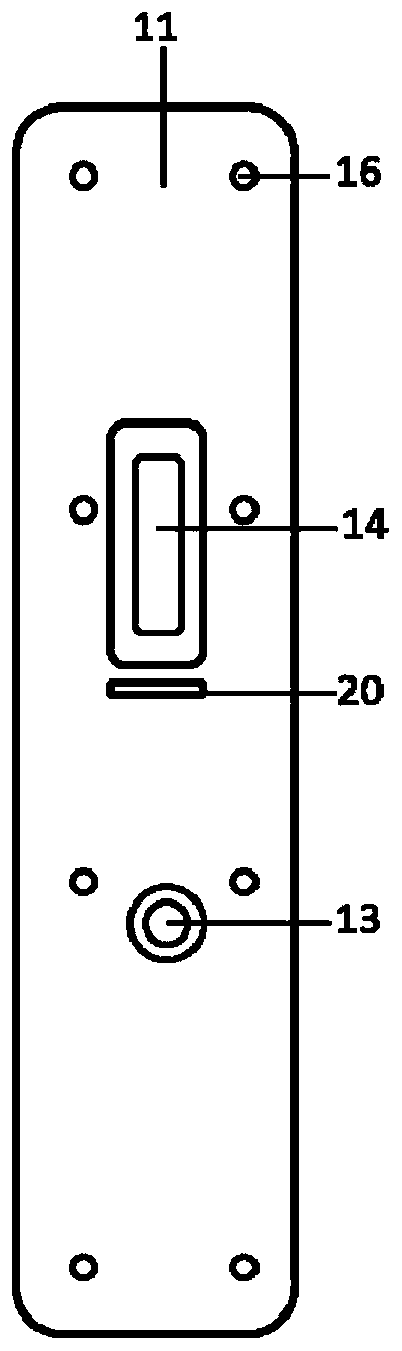
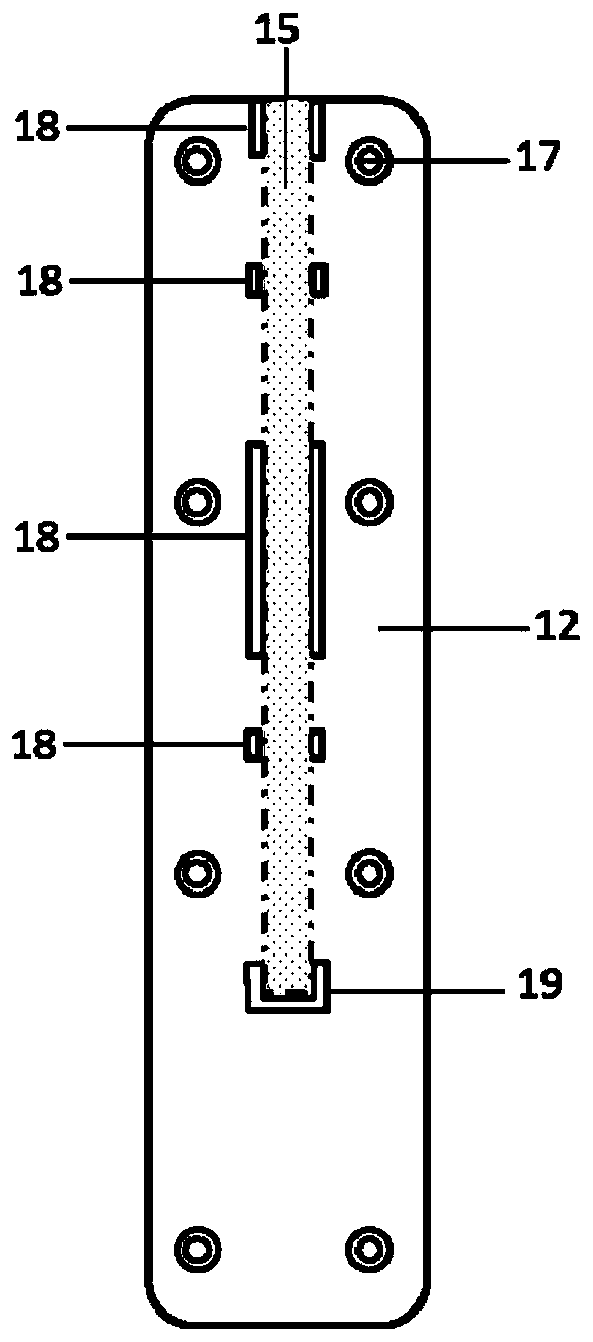
Kit for detecting novel coronavirus N protein and application thereof
A coronavirus and kit technology, applied in the field of kits for detecting the N protein of the new coronavirus, can solve the problems of insufficiently meeting the needs of epidemic prevention and control, and restricting the supply capacity of the rapid detection service of the new coronavirus, so as to reduce social panic. Emotion, fast detection speed, and the effect of accelerated screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Preparation of novel coronavirus nucleocapsid (N) protein immunochromatographic detection kit
[0046] 1) Use fluorescent microspheres to label another strain of mouse anti-new coronavirus N protein monoclonal antibody
[0047] Take 0.5mL fluorescent microspheres, add 1mg carbodiimide (EDC), 1mg N-hydroxysuccinimide (NHS), stir at room temperature, 120r / min for 3h, then add 100μL of another strain of mouse anti-new coronavirus For virus N protein monoclonal antibody, stir for 1 hour at room temperature at a speed of 120 r / min, then add 10 mg of BSA blocking solution, and continue stirring for 1 hour at a speed of 120 r / min. Centrifuge at 12000 r / min for 20 min at 2-8 °C, and remove the supernatant. Finally, the solid precipitate obtained after centrifugation was redissolved to 1 mL with 0.2 M phosphate buffer (pH=7.4), and then 1 μL of Proclin300 was added and stored at 4°C until use.
[0048] 2) Using fluorescent microspheres to label rabbit IgG polyclonal antibod...
Embodiment 2
[0077] 1. Nasal / oropharyngeal swab sample testing
[0078] The types of samples taken included nasopharyngeal swabs and oropharyngeal swabs.
[0079] After the nasal / oropharyngeal swab samples are collected, they are pre-treated with the sample preservation solution (see Table 3 for the composition). Store in solution and stir. Squeeze the outside of the plastic hose several times with your fingers to fully soak the cotton swab with the sample preservation solution, then pull out the cotton swab, and the liquid that is wrung out (that is, the sample collected on the cotton swab is mixed into the sample preservation solution) is the sample to be tested . Then take 60 μL of the processed sample to be tested and drop it into the sample hole of the test card, let it stand for 15 minutes, and then insert it into the fluorescence immunochromatography analyzer for detection, the instrument automatically calculates the T / C value of the sample, and passes the normal range Judge nega...
Embodiment 3
[0087] Example 3 Serum / Plasma / Whole Blood Sample Detection
[0088] The types of samples taken include serum / plasma / whole blood.
[0089] After the serum / plasma / whole blood sample is collected, no sample pretreatment is required, and the sample to be tested is directly added dropwise to the sample inlet of the test card and allowed to stand for 15 minutes, and then inserted into the fluorescence immunochromatography analyzer for detection, the instrument automatically calculates the sample T / C value, judge negative or positive by comparing with normal value range. In addition, when two fluorescent bands appear under ultraviolet light irradiation, it is positive. The clinical test results are shown in Table 5 below.
[0090] table 5
[0091]
[0092]
[0093] As can be seen from the clinical test data in Table 5, all 20 negative samples were detected as negative, which was consistent with the clinical diagnosis; all 20 positive samples were tested positive, with a posi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com