A kind of preparation method of (2s,3s)-2-hydroxyl-4-phenylbutane derivative
A technology of phenylbutane and its derivatives, which is applied in the field of bio-enzyme catalysis, can solve the problems of increasing the contact area between the substrate and the enzyme, the product purity is only 99%, and the difficulty of product separation and purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
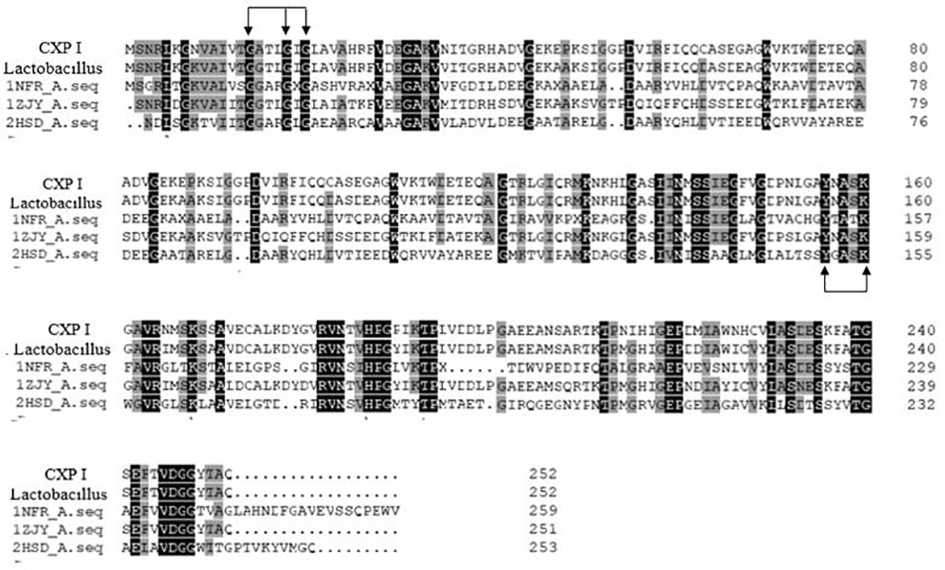
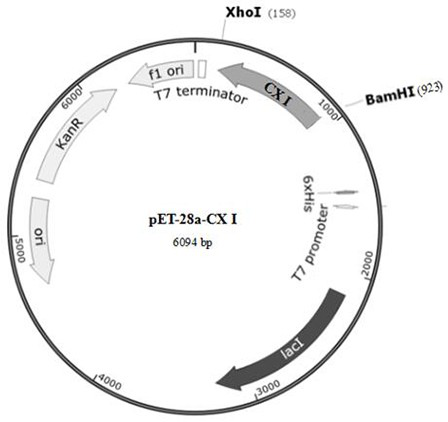
[0043] Carbonyl reductase CXP I, whose amino acid sequence is shown in SEQ ID No.1, contains a polypeptide chain consisting of 253 amino acids, and is obtained by performing site-directed mutation on the gene of CX-LAC II strain.
[0044] The CX-LAC II strain was preserved in the China Type Culture Collection Center (CGMCC) on February 24, 2020, with the preservation number NO.19432, and was identified as Lactobacillus glutinum ( Lactobacillus farraginis ), facultative anaerobic, in the MRS solid medium, the colony is raised, slightly white, moist, with neat edges, and the colony is round, with a diameter of 3.0 mm±1.0 mm. Microscopically, Gram-positive, generally short rods arranged in chains, usually immobile. CX-LAC II contains gene sequence CX II as shown in SEQID No.3.
[0045] Conduct site-directed mutation design on the gene sequence of CX II, mutate the 24th base A to T, the 519th base T to A, the 568-570th base TAT to CCG, and the 678th base The base T at position...
Embodiment 2
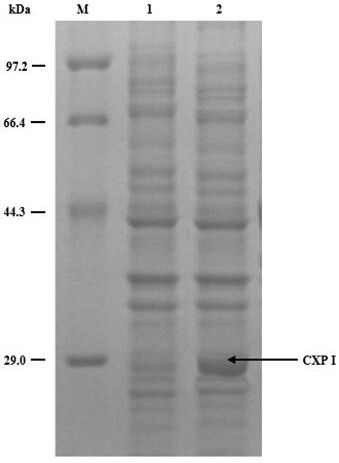
[0051] With (3S)-3-(tert-butoxycarbonyl)amino-1-chloro-4-phenyl-2-butanone as a substrate, the crude enzyme solution of carbonyl reductase obtained in Example 1 was used for enzyme activity experiments, and the preparation The reaction system is as follows: 50 mL system: add 1g (3S)-3-(tert-butoxycarbonyl)amino-1-chloro-4-phenyl-2-butanone, add different concentrations of isopropanol, stir at 40°C, add 2.4 g magnesium sulfate, 130 mg NAD + , use potassium phosphate or sodium buffer solution (100 mmol / L, pH8.0) to make up to 50 mL, and add 1 g of crude enzyme solution. After reacting for 24 hours, the conversion rate and de value were detected by high performance liquid chromatography (HPLC). The results are shown in Table 1. It can be seen that CXP I can be normally converted to almost exhausted substrate under 60% (m / V) isopropanol concentration, and the product de value is higher. Although general carbonyl reductases also exhibit excellent de values, their activity decreas...
Embodiment 3
[0055] The present invention increases the input amount of isopropanol in the system by providing a carbonyl reductase that is tolerant to high-concentration alcohol solutions. As an excellent substrate solvent and a hydrogen donor for the regeneration cycle of cofactors, it can further improve the substrate yield. Concentration, promote the conversion rate to break through the limit of the existing technology, can be further improved.
[0056] In a 1L catalytic system, add 80g (3S)-3-(tert-butoxycarbonyl)amino-1-chloro-4-phenyl-2-butanone, 2.4g magnesium sulfate, 60g glucose, 130 mg NAD +, and then adjust the pH to 7.0 with sodium carbonate solution, stir at 40° C., add 20 g of glucose dehydrogenase, and 4 g of the carbonyl reductase CXP I crude enzyme solution obtained in Example 1 above to start the reaction. Sodium carbonate solution was used for the reaction to maintain a pH of about 7.5. After 40 hours of reaction, 70% of the residual acid substrate was detected in the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallinity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com