Method for heterologous production of linear triterpenes
A linear, triterpenoid technology, applied in the field of bioengineering, can solve the problems of immature genetic manipulation and research lag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Construction of recombinant yeast strains
[0030] Construction of yeast strains expressing CYP genes includes:
[0031] Using the cDNA library of Ganoderma lucidum 5.616 as a template, the coding region sequence (CDS) fragment of cyp505d13 was obtained by PCR amplification with primer GL17184-F / R. The specific sequences of the primers are shown in Sequence Table 1;
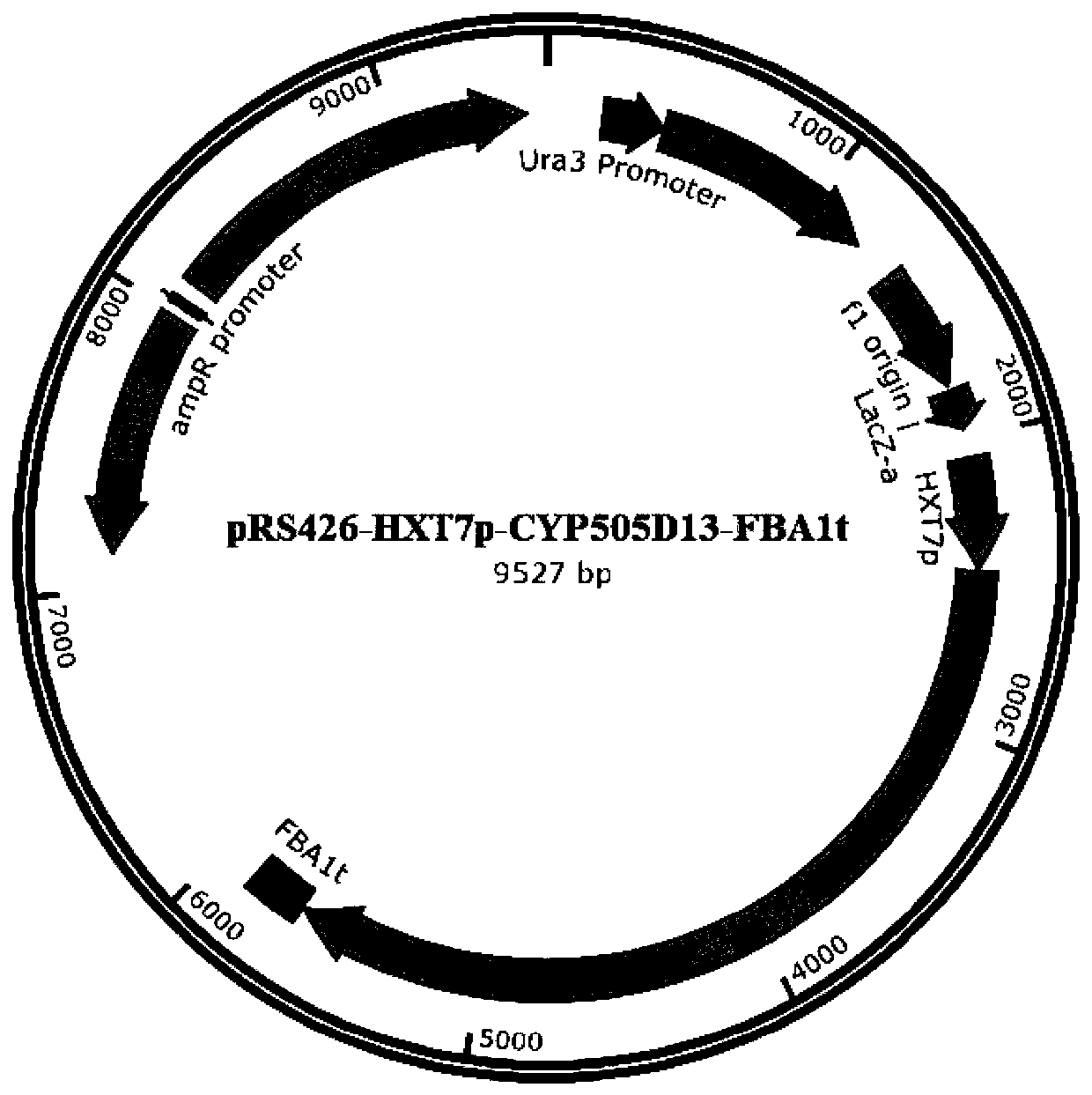
[0032] Subsequently, the CDS fragment was recombined into the expression vector pRS426 and verified by sequencing. The recombinant plasmid pRS426HF-cyp505d13 was obtained;
[0033] The recombinant plasmid was transformed into yeast strain YL-T3, thereby obtaining yeast strain YL-T3-cyp505d13 expressing CYP gene.
[0034] The recombinant plasmid pRS426HF-CYP17184 is specifically obtained in the following manner:
[0035] i) The pRS426 plasmid was linearized by SmaI, and then the yeast endogenous HXT7p promoter and FBA1t terminator were introduced at both ends thereof.
[0036] The HXT7p promoter and FBA1t...
Embodiment 2
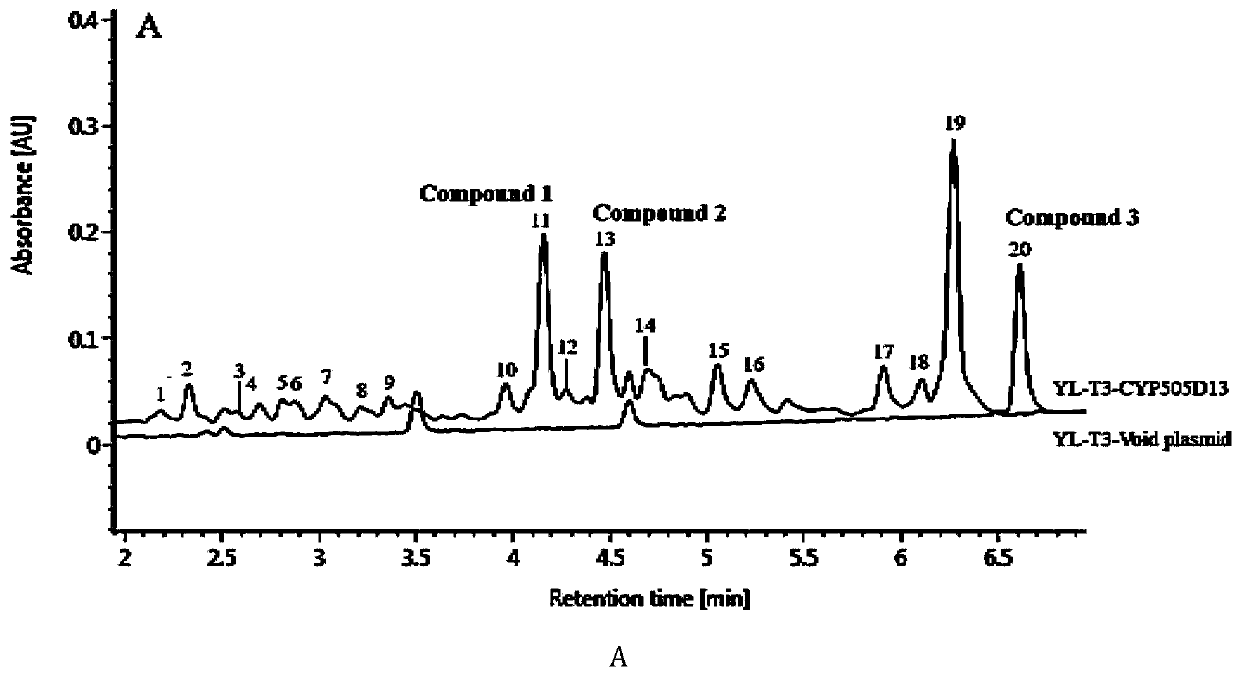
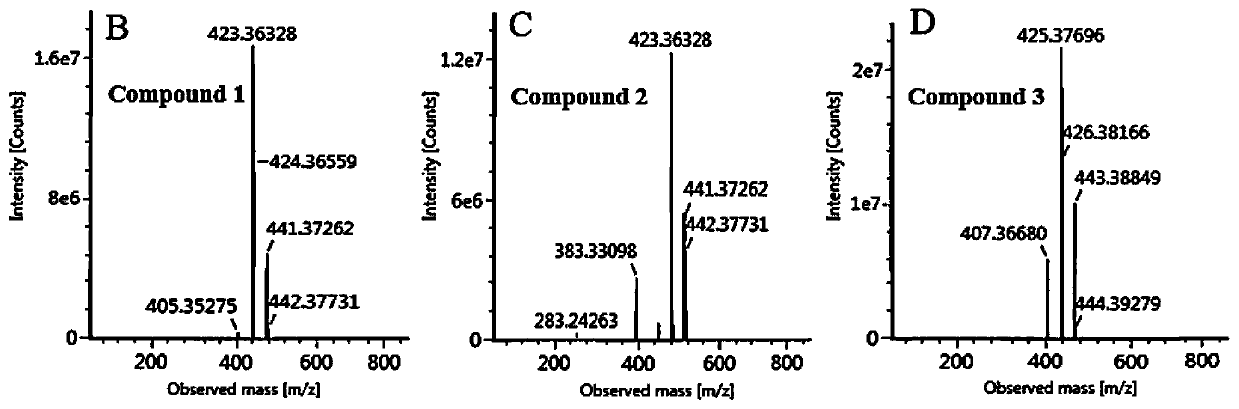
[0046] Product Identification of Fermentation YL-T3-cyp505d13
[0047] Fermentation of YL-T3-cyp505d13: 2.1) Fermentation of YL-T3-cyp505d13 yeast transformants in YPD medium (1% yeast powder, 2% beef peptone, 2% glucose), and use empty plasmid without CYP gene The strains were used as controls, and the differences in metabolites between them were compared, and the specific operations were as follows:
[0048] 2.1.1) Fermentation culture of transformants. Inoculate positive transformants into 4mL SC-HLU seed culture test tubes, culture at 30°C, 220rpm for about 30h, absorb 1mL of cultured seeds, transfer to 50mL of YPD medium, and ferment at 30°C, 220rpm.
[0049] 2.1.2) On the fourth day of fermentation culture, take 20 mL of fermentation broth and add 20 mL of ethyl acetate to shake at 220 rpm for 30 min. Centrifuge at 5000rpm for 5min and absorb the upper organic phase, transfer to a rotary evaporator, spin dry ethyl acetate at 40°C at -0.09Mpa on a rotary evaporator, add...
Embodiment 3
[0052] Determination and Dynamic Accumulation Process of Fermentation Products of Yeast Transformants
[0053] 3.1) High performance liquid chromatography analysis method of fermentation product:
[0054] Instrument: Agilent 1200 Analytical HPLC; Chromatographic column: Agilent ZORBAX SB C18 reverse-phase chromatographic column (5um, 4.6x250mm)
[0055] Column temperature: 30°C; Flow rate: 1mL / min; Injection volume: 20μL;
[0056] Phase A: methanol (containing 0.1% acetic acid), phase B: pure water;
[0057] Gradient elution program: initial phase A 80%, phase B 20%; 0-30min, 80%-100% phase A; 30-40min, 100% phase A. The detection wavelength is 210nm.
[0058] 3.2) The transformed bacterial strain YL-T3-cyp505d13 comprising CYP and the YL-T3 empty plasmid control bacteria were fermented in the YPD medium, and the growth of the two strains (OD 600 ) and the dynamic process of product accumulation such as image 3 shown. The growth conditions of the YL-T3-cyp505d13 strain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com